Diabetes Metab J.
2012 Feb;36(1):37-42. 10.4093/dmj.2012.36.1.37.
Role of HbA1c in the Screening of Diabetes Mellitus in a Korean Rural Community
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. cchung@yonsei.ac.kr
- 2Department of Preventive Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Institute of Lifestyle Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2281427
- DOI: http://doi.org/10.4093/dmj.2012.36.1.37
Abstract
- BACKGROUND
Recently, the measurement of glycated hemoglobin (HbA1c) was recommended as an alternative to fasting plasma glucose or oral glucose tolerance tests for diagnosing diabetes mellitus (DM). In this study, we analyzed HbA1c levels for diabetes mellitus screening in a Korean rural population.
METHODS
We analyzed data from 10,111 subjects from a Korean Rural Genomic Cohort study and generated a receiver operating characteristic curve to determine an appropriate HbA1c cutoff value for diabetes.
RESULTS
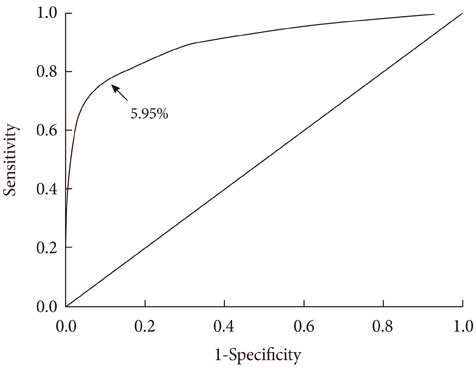
The mean age of the subjects was 56.3+/-8.1 years. Fasting plasma glucose and 2-hour plasma glucose after 75 g oral glucose tolerance tests were 97.5+/-25.6 and 138.3+/-67.1 mg/dL, respectively. The mean HbA1c level of the subjects was 5.7+/-0.9%. There were 8,809 non-DM patients (87.1%) and 1,302 DM patients (12.9%). A positive relationship between HbA1c and plasma glucose levels and between HbA1c and 2-hour plasma glucose levels after oral glucose tolerance tests was found in a scatter plot of the data. Using Youden's index, the proper cutoff level of HbA1c for diabetes mellitus screening was 5.95% (sensitivity, 77%; specificity, 89.4%).
CONCLUSION
Our results suggest that the optimal HbA1c level for DM screening is 5.95%.
MeSH Terms
Figure
Cited by 1 articles
-
Recent advances of medical journals in Korea and and further development strategies: Is it possible for them to publish Nobel Prize-winning research?
Sun Huh
J Korean Med Assoc. 2018;61(9):524-531. doi: 10.5124/jkma.2018.61.9.524.
Reference
-
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998. 21:1414–1431.2. Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008. 93:2447–2453.3. Sung KC, Rhee EJ. Glycated haemoglobin as a predictor for metabolic syndrome in non-diabetic Korean adults. Diabet Med. 2007. 24:848–854.4. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997. 20:1183–1197.5. Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med. 2007. 24:333–343.6. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010. 33:Suppl 1. S62–S69.7. Korean Diabetes Association. Diagnosis and classification of diabetes. Clin Diabetes. 2005. 6:132–140.8. Petersen PH, Jorgensen LG, Brandslund I, De Fine Olivarius N, Stahl M. Consequences of bias and imprecision in measurements of glucose and HbA1c for the diagnosis and prognosis of diabetes mellitus. Scand J Clin Lab Invest Suppl. 2005. 240:51–60.9. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002. 48:436–472.10. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.11. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.12. Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999 2004 NHANES population. Diabetes Care. 2007. 30:2233–2235.13. van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Polak BC. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. 2003. 121:245–251.14. Tapp RJ, Tikellis G, Wong TY, Harper CA, Zimmet PZ, Shaw JE. Australian Diabetes Obesity and Lifestyle Study Group. Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2008. 31:1349–1354.15. Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE. NGSP Steering Committee. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem. 2001. 47:1985–1992.16. Nakagami T, Tominaga M, Nishimura R, Yoshiike N, Daimon M, Oizumi T, Tajima N. Is the measurement of glycated hemoglobin A1c alone an efficient screening test for undiagnosed diabetes? Japan National Diabetes Survey. Diabetes Res Clin Pract. 2007. 76:251–256.17. Kim SY, Park JH, Kang SM, Jin HY, Baek HS, Park TS. Value of HbA1c for diabetic screening in subject with normal fasting glucose. Korean Diabetes J. 2008. 32:Suppl 2. S218.18. Ku YH, Yoo SH, Jung HS, Lim S, Moon MK, Choi SH, Jang HC, Park KS, Kim SY, Lee HK, Cho YM. Diagnostic value of HbA1c different clinical setting with different prevelence of diabetes mellitus. Korean Diabetes J. 2008. 32:Suppl 8. S311.19. Bae JC, Rhee EJ, Choi ES, Kim JH, Kim WJ, Yoo SH, Park SE, Park CY, Lee WY, Oh KW, Park SW, Kim SW. The cutoff value of HbA1c in predicting diabetes in Korean adults in a university hospital in Seoul. Korean Diabetes J. 2009. 33:503–510.20. Jung JH, Kim ST, Cho YZ, Lee HN, Kim JY, Kim JH, Lim DM, Lee KW, Kim BJ, Park KY. Acceptability of HbA1c values as a diagnostic tool for diabetes mellitus in Korea. Korean J Med. 2010. 79:673–680.21. Engelgau MM, Narayan KM, Herman WH. Screening for type 2 diabetes. Diabetes Care. 2000. 23:1563–1580.22. Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flegal KM, Eberhardt MS, Goldstein DE. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000. 23:187–191.23. Eid WE, Pottala JV. Value of hemoglobin A1c in diagnosing diabetes mellitus within a chronic disease management system illustrated by the receiver operating characteristic curve. Endocr Pract. 2010. 16:14–20.24. Larsen ML. The utility of glycated hemoglobin in identification of impaired glucose tolerance. Diabetes Res. 1989. 12:67–70.25. Verrillo A, de Teresa A, Golia R, Nunziata V. The relationship between glycosylated haemoglobin levels and various degrees of glucose intolerance. Diabetologia. 1983. 24:391–393.26. Weykamp CW, Penders TJ, Miedema K, Muskiet FA, van der Slik W. Standardization of glycohemoglobin results and reference values in whole blood studied in 103 laboratories using 20 methods. Clin Chem. 1995. 41:82–86.27. Korean National Health and Nutrition Examination Survey: the 4th report (2008). Ministry of Health and Welfare; Korea Centers for Disease Control and Prevention. updated 2009 Dec 12. Available from: http://www.bokjiro.go.kr/data/statusView.do?board_sid=297&data_sid=209708&searchSort=REG_DESC&pageIndex=1&searchWrd=%EA%B5%AD%EB%AF%BC%EA%B1%B4%EA%B0%95%ED%86%B5%EA%B3%84&searchCont=&pageUnit=10.28. National Health and Nutrition Examination Survey (NHANES) 2005-2006. Centers for Disease Control and Prevention. updated 2011 Apr 29. Available from: http://www.cdc.gov/nchs/nhanes/nhanes 2005-2006/nhanes05_06.htm.29. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003. 88:2300–2308.30. McCarter RJ, Hempe JM, Chalew SA. Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: an analysis of data from the Diabetes Control and Complications Trial. Diabetes Care. 2006. 29:352–355.31. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E. Diabetes Prevention Program Research Group. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007. 30:2453–2457.32. Selvin E, Zhu H, Brancati FL. Elevated A1C in adults without a history of diabetes in the U.S. Diabetes Care. 2009. 32:828–833.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glycated Hemoglobin and All-Cause Mortality in Korean Type 2 Diabetes
- HbA1c Variability and Micro- and Macrovascular Complications of Diabetes
- The role and responsibility of community health practitioner based on the rural community development and the reform of health care system
- Validity of hemoglobin alc measurement by HPLC as a screening test for diabetes mellitus
- Management Strategies for Children and Adolescents with Diabetes Mellitus