Diabetes Metab J.
2015 Feb;39(1):10-15. 10.4093/dmj.2015.39.1.10.
Hepatokines as a Link between Obesity and Cardiovascular Diseases
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. medica7@gmail.com
- KMID: 2280651
- DOI: http://doi.org/10.4093/dmj.2015.39.1.10
Abstract
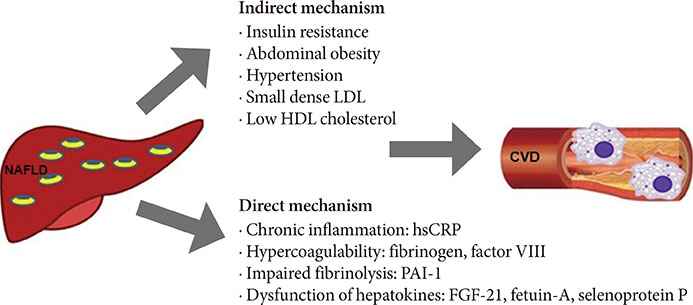
- Non-alcoholic fatty liver disease, which is considered a hepatic manifestation of metabolic syndrome, independently increases the risks of developing cardiovascular disease (CVD) and type 2 diabetes mellitus. Recent emerging evidence suggests that a group of predominantly liver-derived proteins called hepatokines directly affect the progression of atherosclerosis by modulating endothelial dysfunction and infiltration of inflammatory cells into vessel walls. Here, we summarize the role of the representative hepatokines fibroblast growth factor 21, fetuin-A, and selenoprotein P in the progression of CVD.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Close Relationship between Non-Alcoholic Fatty Liver Disease Marker and New-Onset Hypertension in Healthy Korean Adults
Jae-Hyung Roh, Jae-Hyeong Park, Hanbyul Lee, Yong-Hoon Yoon, Minsu Kim, Yong-Giun Kim, Gyung-Min Park, Jae-Hwan Lee, In-Whan Seong
Korean Circ J. 2020;50(8):695-705. doi: 10.4070/kcj.2019.0379.
Reference
-
1. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013; 10:330–344.2. Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012; 33:1190–1200.3. Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014; 34:1155–1161.4. Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH, Santos RD, Budoff MJ, Nasir K. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013; 230:258–267.5. Akabame S, Hamaguchi M, Tomiyasu K, Tanaka M, Kobayashi-Takenaka Y, Nakano K, Oda Y, Yoshikawa T. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT). Circ J. 2008; 72:618–625.6. Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005; 42:473–480.7. Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007; 30:1212–1218.8. Haring R, Wallaschofski H, Nauck M, Dorr M, Baumeister SE, Volzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology. 2009; 50:1403–1411.9. Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007; 132:2103–2115.10. Bjorntorp P. "Portal" adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990; 10:493–496.11. Lavie CJ, Milani RV, Verma A, O'Keefe JH. C-reactive protein and cardiovascular diseases: is it ready for primetime? Am J Med Sci. 2009; 338:486–492.12. Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013; 9:144–152.13. Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010; 139:456–463.14. Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008; 8:169–174.15. Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012; 148:556–567.16. Yu H, Xia F, Lam KS, Wang Y, Bao Y, Zhang J, Gu Y, Zhou P, Lu J, Jia W, Xu A. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem. 2011; 57:691–700.17. Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, Zierath JR, Chibalin AV, Moller DE, Kharitonenkov A, Krook A. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev. 2011; 27:286–297.18. Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013; 18:333–340.19. Dostalova I, Haluzikova D, Haluzik M. Fibroblast growth factor 21: a novel metabolic regulator with potential therapeutic properties in obesity/type 2 diabetes mellitus. Physiol Res. 2009; 58:1–7.20. Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M, Villarroya F. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013; 4:2019.21. Patel V, Adya R, Chen J, Ramanjaneya M, Bari MF, Bhudia SK, Hillhouse EW, Tan BK, Randeva HS. Novel insights into the cardio-protective effects of FGF21 in lean and obese rat hearts. PLoS One. 2014; 9:e87102.22. Lu Y, Liu JH, Zhang LK, Du J, Zeng XJ, Hao G, Huang J, Zhao DH, Wang GZ, Zhang YC. Fibroblast growth factor 21 as a possible endogenous factor inhibits apoptosis in cardiac endothelial cells. Chin Med J (Engl). 2010; 123:3417–3421.23. Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, Tse HF, Chau MT, Cheung BM, Lam KS. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2013; 33:2454–2459.24. Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, Lu Z, Gao M, Bao Y, Jia W. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol. 2013; 12:124.25. Yang SJ, Hong HC, Choi HY, Yoo HJ, Cho GJ, Hwang TG, Baik SH, Choi DS, Kim SM, Choi KM. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin Endocrinol (Oxf). 2011; 75:464–469.26. Nangami GN, Watson K, Parker-Johnson K, Okereke KO, Sakwe A, Thompson P, Frimpong N, Ochieng J. Fetuin-A (alpha2HS-glycoprotein) is a serum chemo-attractant that also promotes invasion of tumor cells through Matrigel. Biochem Biophys Res Commun. 2013; 438:660–665.27. Denecke B, Graber S, Schafer C, Heiss A, Woltje M, Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem J. 2003; 376(Pt 1):135–145.28. Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Haring HU, Stefan N. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One. 2008; 3:e1765.29. Dasgupta S, Bhattacharya S, Biswas A, Majumdar SS, Mukhopadhyay S, Ray S, Bhattacharya S. NF-kappaB mediates lipid-induced fetuin-A expression in hepatocytes that impairs adipocyte function effecting insulin resistance. Biochem J. 2010; 429:451–462.30. Jung TW, Youn BS, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Kim BH, Baik SH, Choi KM. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem Pharmacol. 2013; 86:960–969.31. Ou HY, Yang YC, Wu HT, Wu JS, Lu FH, Chang CJ. Increased fetuin-A concentrations in impaired glucose tolerance with or without nonalcoholic fatty liver disease, but not impaired fasting glucose. J Clin Endocrinol Metab. 2012; 97:4717–4723.32. Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG. Health ABC Study. Fetuin-A and incident diabetes mellitus in older persons. JAMA. 2008; 300:182–188.33. Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006; 113:1760–1767.34. Choi KM, Han KA, Ahn HJ, Lee SY, Hwang SY, Kim BH, Hong HC, Choi HY, Yang SJ, Yoo HJ, Baik SH, Choi DS, Min KW. The effects of caloric restriction on fetuin-A and cardiovascular risk factors in rats and humans: a randomized controlled trial. Clin Endocrinol (Oxf). 2013; 79:356–363.35. Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Haring HU, Schulze MB. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008; 57:2762–2767.36. Mori K, Emoto M, Inaba M. Fetuin-A and the cardiovascular system. Adv Clin Chem. 2012; 56:175–195.37. Evrard S, Delanaye P, Kamel S, Cristol JP, Cavalier E. SFBC/SN joined working group on vascular calcifications. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta. 2015; 438:401–414.38. Jensen MK, Bartz TM, Mukamal KJ, Djousse L, Kizer JR, Tracy RP, Zieman SJ, Rimm EB, Siscovick DS, Shlipak M, Ix JH. Fetuin-A, type 2 diabetes, and risk of cardiovascular disease in older adults: the cardiovascular health study. Diabetes Care. 2013; 36:1222–1228.39. Laughlin GA, Cummins KM, Wassel CL, Daniels LB, Ix JH. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: the Rancho Bernardo study. J Am Coll Cardiol. 2012; 59:1688–1696.40. Siegel-Axel DI, Ullrich S, Stefan N, Rittig K, Gerst F, Klingler C, Schmidt U, Schreiner B, Randrianarisoa E, Schaller HE, Stock UA, Weigert C, Konigsrainer A, Haring HU. Fetuin-A influences vascular cell growth and production of proinflammatory and angiogenic proteins by human perivascular fat cells. Diabetologia. 2014; 57:1057–1066.41. Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr. 2005; 25:215–235.42. Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, Sakurai M, Yamashita T, Mizukoshi E, Yamashita T, Honda M, Miyamoto K, Kubota T, Kubota N, Kadowaki T, Kim HJ, Lee IK, Minokoshi Y, Saito Y, Takahashi K, Yamada Y, Takakura N, Kaneko S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010; 12:483–495.43. Choi HY, Hwang SY, Lee CH, Hong HC, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic Fatty liver disease. Diabetes Metab J. 2013; 37:63–71.44. Yang SJ, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab. 2011; 96:E1325–E1329.45. Jung TW, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Youn BS, Baik SH, Choi KM. Salsalate and adiponectin improve palmitate-induced insulin resistance via inhibition of selenoprotein P through the AMPK-FOXO1alpha pathway. PLoS One. 2013; 8:e66529.46. Ishikura K, Misu H, Kumazaki M, Takayama H, Matsuzawa-Nagata N, Tajima N, Chikamoto K, Lan F, Ando H, Ota T, Sakurai M, Takeshita Y, Kato K, Fujimura A, Miyamoto K, Saito Y, Kameo S, Okamoto Y, Takuwa Y, Takahashi K, Kidoya H, Takakura N, Kaneko S, Takamura T. Selenoprotein P as a diabetes-associated hepatokine that impairs angiogenesis by inducing VEGF resistance in vascular endothelial cells. Diabetologia. 2014; 57:1968–1976.