Korean J Pain.
2012 Oct;25(4):228-237. 10.3344/kjp.2012.25.4.228.
Evaluation of the Neurological Safety of Epidural Milnacipran in Rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chung-Ang University College of Medicine, Seoul, Korea. pain@cau.ac.kr
- 2Department of Prosthodontics, Dentistry, Hangang Sacred Heart Hospital, Graduated School of Clinical Dentistry, Hallym University, Seoul, Korea.
- 3Department of Surgery, Chung-Ang University College of Medicine, Seoul, Korea.
- 4Department of Anesthesiology and Pain Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Department of Pathology, Korea University College of Medicine, Seoul, Korea.
- 6Department of Anesthesiology and Pain Medicine, Seoul National University Hospital, Seoul, Korea.
- KMID: 2278163
- DOI: http://doi.org/10.3344/kjp.2012.25.4.228
Abstract
- BACKGROUND
Milnacipran is a balanced serotonin norepinephrine reuptake inhibitor with minimal side effects and broad safety margin. It acts primarily on the descending inhibitory pain pathway in brain and spinal cord. In many animal studies, intrathecal administration of milnacipran is effective in neuropathic pain management. However, there is no study for the neurological safety of milnacipran when it is administered neuraxially. This study examined the neurotoxicity of epidural milnacipran by observing behavioral and sensory-motor changes with histopathological examinations of spinal cords in rats.
METHODS
Sixty rats were divided into 3 groups, with each group receiving epidural administration of either 0.3 ml (3 mg) of milnacipran (group M, n = 20), 0.3 ml of 40% alcohol (group A, n = 20), or 0.3 ml of normal saline (group S, n = 20).
RESULTS
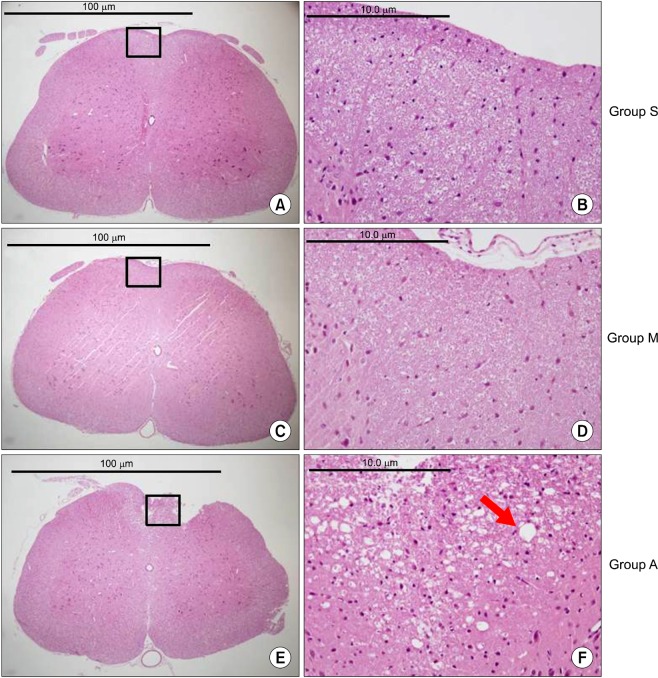
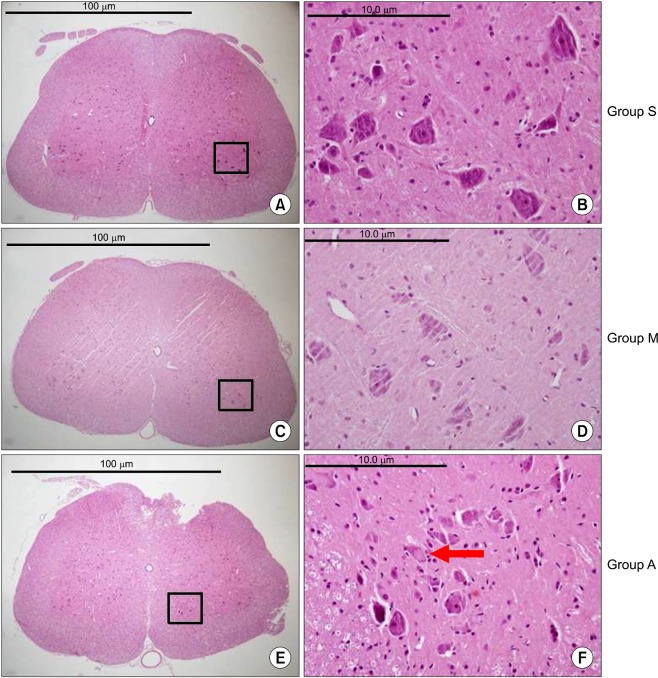
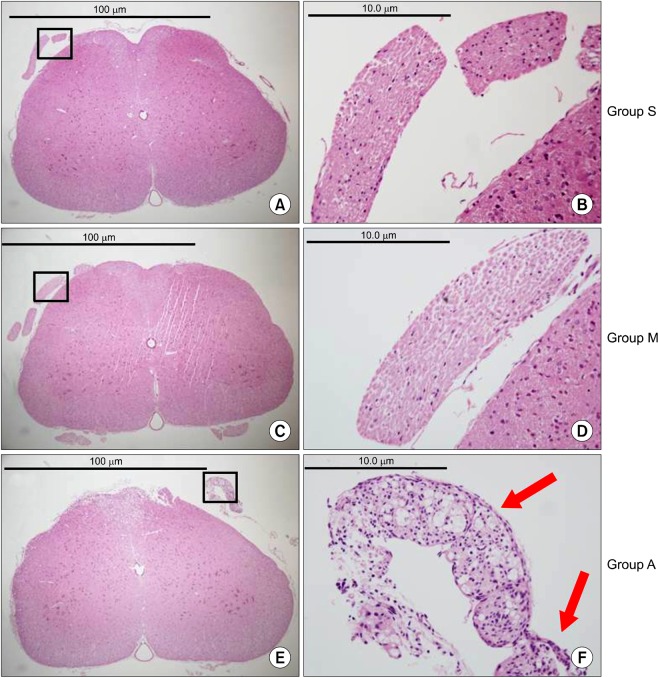
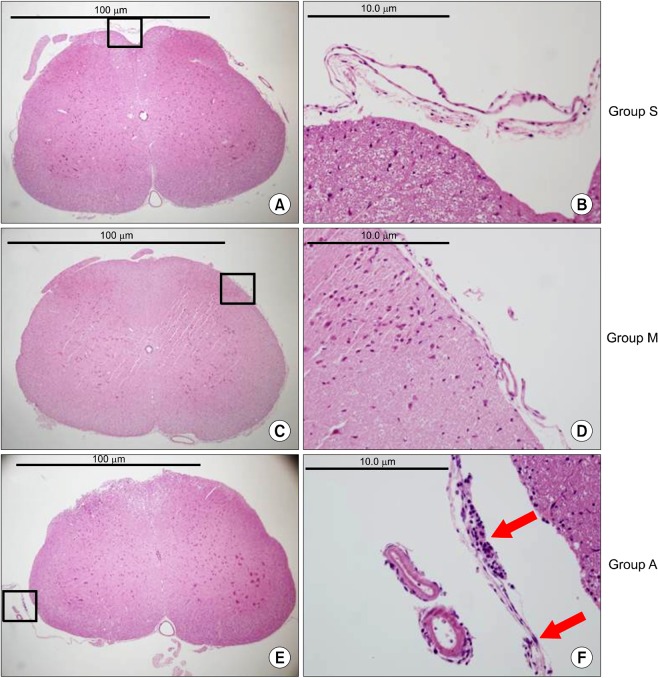
There were no abnormal changes in the behavioral, sensory-motor, or histopathological findings in all rats of groups M and S over a 3-week observation period, whereas all rats in group A had abnormal changes.
CONCLUSIONS
Based on these findings, the direct epidural administration of milnacipran in rats did not present any evidence of neurotoxicity in behavioral, sensory-motor and histopathological evaluations.
Keyword
MeSH Terms
Figure
Reference
-
1. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999; 83:389–400. PMID: 10568846.
Article2. Walsh TD. Antidepressants in chronic pain. Clin Neuropharmacol. 1983; 6:271–295. PMID: 6141005.
Article3. Obata H, Saito S, Koizuka S, Nishikawa K, Goto F. The monoamine-mediated antiallodynic effects of intrathecally administered milnacipran, a serotonin noradrenaline reuptake inhibitor, in a rat model of neuropathic pain. Anesth Analg. 2005; 100:1406–1410. PMID: 15845695.
Article4. Fanton L, Bévalot F, Grait H, Le Meur C, Gaillard Y, Malicier D. Fatal intoxication with milnacipran. J Forensic Leg Med. 2008; 15:388–390. PMID: 18586210.
Article5. Ikeda T, Ishida Y, Naono R, Takeda R, Abe H, Nakamura T, et al. Effects of intrathecal administration of newer antidepressants on mechanical allodynia in rat models of neuropathic pain. Neurosci Res. 2009; 63:42–46. PMID: 18992286.
Article6. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol. 2004; 19(Suppl 1):S15–S19. PMID: 15378668.
Article7. Shin SW, Eisenach JC. Peripheral nerve injury sensitizes the response to visceral distension but not its inhibition by the antidepressant milnacipran. Anesthesiology. 2004; 100:671–675. PMID: 15108984.
Article8. Park SC, Shin SW, Baek SH, Kim HK, Baik SW, Kim KH. The effects of milnacipran on rat pain model. Korean J Anesthesiol. 2004; 47:260–265.
Article9. Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003; 55:1007–1041. PMID: 12935942.
Article10. Yaksh TL, Collins JG. Studies in animals should precede human use of spinally administered drugs. Anesthesiology. 1989; 70:4–6. PMID: 2912314.
Article11. Schug SA, Saunders D, Kurowski I, Paech MJ. Neuraxial drug administration: a review of treatment options for anaesthesia and analgesia. CNS Drugs. 2006; 20:917–933. PMID: 17044729.12. Kim YC, Lim YJ, Lee SC. Spreading pattern of epidurally administered contrast medium in rabbits. Acta Anaesthesiol Scand. 1998; 42:1092–1095. PMID: 9809094.
Article13. Lim YJ, Sim WS, Kim YC, Lee SC, Choi YL. The neurotoxicity of epidural hyaluronic acid in rabbits: a light and electron microscopic examination. Anesth Analg. 2003; 97:1716–1720. PMID: 14633548.
Article14. Choi SS, Kim YC, Lim YJ, Lee CJ, Lee PB, Lee SC, et al. The neurological safety of epidural gabapentin in rats: a light microscopic examination. Anesth Analg. 2005; 101:1422–1426. PMID: 16244005.
Article15. Hayashi N, Weinstein JN, Meller ST, Lee HM, Spratt KF, Gebhart GF. The effect of epidural injection of betamethasone or bupivacaine in a rat model of lumbar radiculopathy. Spine (Phila Pa 1976). 1998; 23:877–885. PMID: 9580954.
Article16. Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Tamaki T. Epidural injection of cyclooxygenase-2 inhibitor attenuates pain-related behavior following application of nucleus pulposus to the nerve root in the rat. J Orthop Res. 2002; 20:376–381. PMID: 11918320.
Article17. Lee JR. Evaluation of the neurological safety of epidural pregabalin in rats (dissertation). 2008. Seoul: Seoul National Univ..18. Bajrović F, Sketelj J. Extent of nociceptive dermatomes in adult rats is not primarily maintained by axonal competition. Exp Neurol. 1998; 150:115–121. PMID: 9514823.
Article19. Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995; 82:1013–1025. PMID: 7717536.
Article20. Kawakami M, Weinstein JN, Spratt KF, Chatani K, Traub RJ, Meller ST, et al. Experimental lumbar radiculopathy. Immunohistochemical and quantitative demonstrations of pain induced by lumbar nerve root irritation of the rat. Spine (Phila Pa 1976). 1994; 19:1780–1794. PMID: 7526474.
Article21. Guevara-López U, Covarrubias-Gómez A, Gutierrez-Acar H, Aldrete JA, López-Muñoz FJ, Martínez-Benítez B. Chronic subarachnoid administration of 1-(4chlorobenzoyl)-5methoxy-2methyl-1H-indole-3 acetic acid (indomethacin): an evaluation of its neurotoxic effects in an animal model. Anesth Analg. 2006; 103:99–102. PMID: 16790634.
Article22. Madsen JB, Jensen FM, Faber T, Bille-Hansen V. Chronic catheterization of the epidural space in rabbits: a model for behavioural and histopathological studies. Examination of meptazinol neurotoxicity. Acta Anaesthesiol Scand. 1993; 37:307–313. PMID: 8517109.
Article23. Gurun MS, Leinbach R, Moore L, Lee CS, Owen MD, Eisenach JC. Studies on the safety of glucose and paraben-containing neostigmine for intrathecal administration. Anesth Analg. 1997; 85:317–323. PMID: 9249107.
Article24. Hodgson PS, Neal JM, Pollock JE, Liu SS. The neurotoxicity of drugs given intrathecally (spinal). Anesth Analg. 1999; 88:797–809. PMID: 10195528.
Article25. Fazakas J, Tóth S, Füle B, Smudla A, Mándli T, Radnai M, et al. Epidural anesthesia? No of course. Transplant Proc. 2008; 40:1216–1217. PMID: 18555151.
Article26. Governale LS, Fein N, Logsdon J, Black PM. Techniques and complications of external lumbar drainage for normal pressure hydrocephalus. Neurosurgery. 2008; 63:379–384. PMID: 18981847.
Article27. Hassenbusch SJ, Portenoy RK, Cousins M, Buchser E, Deer TR, Du Pen SL, et al. Polyanalgesic Consensus Conference 2003: an update on the management of pain by intraspinal drug delivery-- report of an expert panel. J Pain Symptom Manage. 2004; 27:540–563. PMID: 15165652.
Article28. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22:659–661. PMID: 17942826.
Article29. Rocco AG, Chan V, Iacobo C. An algorithm for the treatment of pain in advanced cancer. Hosp J. 1989; 5:93–103. PMID: 2628254.
Article30. Hogan QH, Stadnicka A, Stekiel TA, Bosnjak ZJ, Kampine JP. Mechanism of mesenteric venodilatation after epidural lidocaine in rabbits. Anesthesiology. 1994; 81:939–945. PMID: 7943844.
Article31. Svensson BA, Alari L, Post C. Repeated intrathecal injections of dezocine produce antinociception without evidence for neurotoxicity in the rat: a study of morphometric evaluation of spinal cord histology. Anesth Analg. 1992; 75:392–399. PMID: 1510261.32. Norton S. Is behavior or morphology a more sensitive indicator of central nervous system toxicity? Environ Health Perspect. 1978; 26:21–27. PMID: 363417.
Article33. Singler RC. Alcohol neurolysis of sciatic and femoral nerves. Anesth Analg. 1981; 60:532–533. PMID: 7195671.
Article34. Porges P, Zdrahal F. Intrathecal alcohol neurolysis of the lower sacral roots in inoperable rectal cancer. Anaesthesist. 1985; 34:627–629. PMID: 2418708.35. Pélissier J, Viel E, Enjalbert M, Kotzki N, Eledjam JJ. Chemical neurolysis using alcohol (alcoholization) in the treatment of spasticity in the hemiplegic. Cah Anesthesiol. 1993; 41:139–143. PMID: 8504349.36. Gallager HS, Yonezawa T, Hay RC, Derrick WS. Subarachnoid alcohol block. II. Histologic changes in the central nervous system. Am J Pathol. 1961; 38:679–693. PMID: 13703113.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Milnacipran on Rat Pain Model
- The Combined Antiallodynic Effect of Gabapentin and Milnacipran in a Rat Neuropathic Pain Model
- Effect of the Combined Use of Tramadol and Milnacipran on Pain Threshold in an Animal Model of Fibromyalgia
- The Neurological Safety of Epidural Pamidronate in Rats
- Evaluation of the neurological safety of epidurally-administered pregabalin in rats