Chonnam Med J.
2012 Apr;48(1):52-56. 10.4068/cmj.2012.48.1.52.
Outcome Evaluation of Intravenous Infusion of Urokinase for Acute Ischemic Stroke
- Affiliations
-
- 1Department of Neurosurgery, Kwangju Christian Hospital, Gwangju, Korea.
- 2Department of Pathology, Chonnam National University Medical School, Gwangju, Korea. lee@jnu.ac.kr
- KMID: 2274882
- DOI: http://doi.org/10.4068/cmj.2012.48.1.52
Abstract
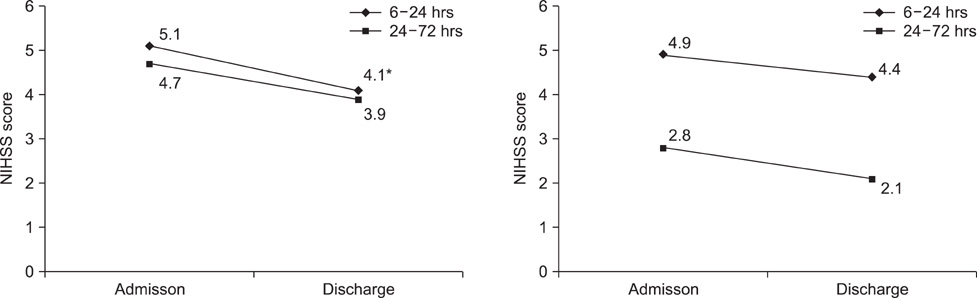
- The aim of this study was to evaluate the clinical effect of a continuous infusion of urokinase in cerebral stoke patients who were late admitted over 6 hours after onset. From January to December in 2008, acute cerebral stroke patients (n=143) treated with intravenous urokinase infusion (Group I, n=93) or not (Group II, n=50) after 6 hours and within 72 hours of stroke onset were reviewed. Continuous intravenous infusion of urokinase was done for 5 days. The clinical outcome for each patient was evaluated by using the modified National Institutes of Health Stroke Scale (NIHSS) on admission and on the day of discharge. The NIHSS score was decreased at discharge compared with admission in the urokinase treatment group (Group I; from 4.8+/-2.2 to 3.8+/-1.9; p=0.002). There was an improvement in the patients who initiated urokinase treatment within 24 hours from stroke onset in Group I (from 5.1+/-1.9 to 3.9+/-1.5; p=0.04). In patients with initiated urokinase treatment within 24 hours from stroke onset, intravenous urokinase infusion could be an effective modality in acute ischemic stroke patients admitted later than 6 hours after onset.
MeSH Terms
Figure
Reference
-
1. Doh WB, Lee BC, Lee IH, Kim SM, Kwon KH. Safety and effect of continuous intravenous urokinase therapy in acute ischemic stroke (Open Clinical Trial). J Korean Neurol Assoc. 1999. 17:189–194.2. Lee JH, Kim JS, Lee MC, Seo DC, Lee MS. A comparison study on therapeutic efficacy of urokinase vs. heparin in acute ischemic stroke. J Korean Neurol Assoc. 1994. 12:225–236.3. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007. 369:275–282.
Article4. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995. 333:1581–1587.5. Chang DI, Yoon SS, Shin WC, Chung KC. Intravenous rt-PA fibrinolytic therapy in acute carotid territory ischemic stroke with severe neurologic deficits: the implication of arterial recanalization for stroke outcome. J Korean Neurol Assoc. 2003. 21:1–6.6. Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. DIAS Study Group. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005. 36:66–73.
Article7. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. American Stroke Association Stroke Council. Clinical Cardiology Council. Cardiovascular Radiology and Intervention Council. Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007. 38:1655–1711.
Article8. von Kummer R, Hacke W. Safety and efficacy of intravenous tissue plasminogen activator and heparin in acute middle cerebral artery stroke. Stroke. 1992. 23:646–652.
Article9. Slivka A, Levy D. Natural history of progressive ischemic stroke in a population treated with heparin. Stroke. 1990. 21:1657–1662.
Article10. Mori E, Tabuchi M, Yoshida T, Yamadori A. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke. 1988. 19:802–812.
Article11. Overgaard K, Sperling B, Boysen G, Pedersen H, Gam J, Ellemann K, et al. Thrombolytic therapy in acute ischemic stroke. A Danish pilot study. Stroke. 1993. 24:1439–1446.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Hemoperitoneum after Intraarterial Urokinase Infusion for Acute Ischemic Stroke
- Safety and Effect of Continuous Intravenous Urokinase Therapy in Acute Ischemic Stroke ( Open Clinical Trial )
- Thrombolytic Treatment for Acute Ischemic Cerebral Stroke: Intraarterial Urokinase Infusion vs. Intravenous Heparin and Urokinase Infusion
- Superselective Local Infusion of Urokinase for Acute Ischemic Stroke in the Carotid Artery Territory
- Treatment of Acute Ischemic Stroke: Thrombolysis