Korean J Orthod.
2008 Oct;38(5):347-357. 10.4041/kjod.2008.38.5.347.
The effects of ipriflavone on the periodontal reorganization following experimental tooth movement in the rat
- Affiliations
-
- 1Department of Orthodontics, School of Dentistry, Chonnam National University, Korea.
- 2Department of Orthodontics, 2nd Stage of Brain Korea 21, School of Dentistry, Dental Science Research Institute, Chonnam National University, Korea. hhwang@chonnam.ac.kr
- KMID: 2273921
- DOI: http://doi.org/10.4041/kjod.2008.38.5.347
Abstract
OBJECTIVE
The purpose of this study was to examine the effect of ipriflavone on periodontal reorganization and prevention of relapse following tooth movement.
METHODS
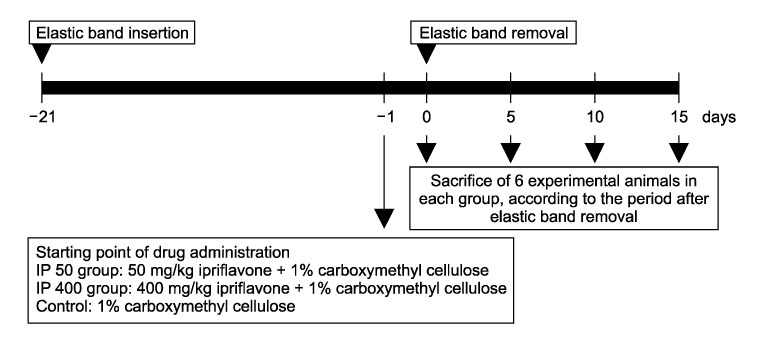
Orthodontic rubber bands were inserted between the first and second maxillary molars of 27 white male rats for 3 weeks for experimental tooth movement. From one day before the removal of orthodontic rubber band, ipriflavone was administered 50 or 400 mg/kg daily in each experimental group whereas carboxymethyl cellulose solution was administered in the control group. They were sacrificed at the 5, 10, and 15th day from the day of removal of orthodontic rubber bands. The amount of relapse was evaluated by measuring the interdental space, and the extent of periodontal reorganization was compared through histological examination.
RESULTS
In case of ipriflavone administration, the amount and velocity of relapse was less and slower compared to the control group. In addition, the ipriflavone group showed more rapid periodontal reorganization compared to the control group.
CONCLUSIONS
The results of the present study suggest that ipriflavone administration can be used effectively in the prevention of relapse following orthodontic tooth movement through the acceleration of periodontal reorganization.
MeSH Terms
Figure
Cited by 1 articles
-
Changes in pulpal blood flow during orthodontic tooth movement studied by Doppler ultrasound
Kyoung-Sub Lim, Young-Min Bae, Jung-Yul Cha, Hyung-Seog Yu, Chung-Ju Hwang
Korean J Orthod. 2009;39(6):372-382. doi: 10.4041/kjod.2009.39.6.372.
Reference
-
1. Reitan K. Principles of retention and avoidance of posttreatment relapse. Am J Orthod. 1969. 55:776–790.
Article2. Edwards JG. A long-term prospective evaluation of the circumferential supracrestal fiberotomy in alleviating orthodontic relapse. Am J Orthod Dentofacial Orthop. 1988. 93:380–387.
Article3. Proffit WR. Proffit WR, Fields HW, editors. Retention. Contemporary Orthodontics. 2000. 3rd ed. St Louis: Mosby;597–614.4. Baumrind S. A reconsideration of the propriety of the "pressure-tension" hypothesis. Am J Orthod. 1969. 55:12–22.
Article5. Davidovitch Z, Finkelson MD, Steigman S, Shanfeld JL, Montgomery PC, Korostoff E. Electric currents, bone remodeling, and orthodontic tooth movement, II. Increase in rate of tooth movement and periodontal cyclic nucleotide levels by combined force and electric current. Am J Orthod. 1980. 77:33–47.6. Reitan K. Tissue behavior during orthodontic tooth movement. Am J Orthod. 1960. 46:881–900.
Article7. Reitan K, Kvam E. Comparative behavior of human and animal tissue during experimental tooth movement. Angle Orthod. 1971. 41:1–14.8. Joondeph DR, Riedel RA. Graber TM, Vanarsdall RL, editors. Retention and Relapse. Orthodontics: Current Principles and Techniques. 1994. St Louis: Mosby-Year Book;908–950.9. Saito M, Saito S, Ngan PW, Shanfeld J, Davidovitch Z. Interleukin 1 beta and prostaglandin E are involved in the response of periodontal cells to mechanical stress in vivo and in vitro. Am J Orthod Dentofacial Orthop. 1991. 99:226–240.
Article10. Binderman I, Zor U, Kaye AM, Shimshoni Z, Harell A, Sömjen D. The transduction of mechanical force into biochemical events in bone cells may involve activation of phospholipase A2. Calcif Tissue Int. 1988. 42:261–266.
Article11. Samuelsson B, Granström E, Green K, Hamberg M, Hammarström S. Prostaglandins. Annu Rev Biochem. 1975. 44:669–695.
Article12. Norevall LI, Forsgren S, Matsson L. Expression of neuropeptides (CGRP, substance P) during and after orthodontic tooth movement in the rat. Eur J Orthod. 1995. 17:311–325.
Article13. Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Orthodontic movement induces high numbers of cells expressing IFN-gamma at mRNA and protein levels. J Interferon Cytokine Res. 2000. 20:7–12.
Article14. Adachi H, Igarashi K, Mitani H, Shinoda H. Effects of topical administration of a bisphosphonate (risedronate) on orthodontic tooth movements in rats. J Dent Res. 1994. 73:1478–1484.
Article15. Giunta D, Keller J, Nielsen FF, Melsen B. Influence of indomethacin on bone turnover related to orthodontic tooth movement in miniature pigs. Am J Orthod Dentofacial Orthop. 1995. 108:361–366.
Article16. Kehoe MJ, Cohen SM, Zarrinnia K, Cowan A. The effect of acetaminophen, ibuprofen, and misoprostol on prostaglandin E2 synthesis and the degree and rat of orthodontic tooth movement. Angle Orthod. 1996. 66:339–349.17. Wong A, Reynolds EC, West VC. The effect of acetylsalicylic acid on orthodontic tooth movement in the guinea pig. Am J Orthod Dentofacial Orthop. 1992. 102:360–365.
Article18. Kim TW, Yoshida Y, Yokoya K, Sasaki T. An ultrastructural study of the effects of bisphosphonate administration on osteoclastic bone resorption during relapse of experimentally moved rat molars. Am J Orthod Dentofacial Orthop. 1999. 115:645–653.
Article19. Igarashi K, Mitani H, Adachi H, Shinoda H. Anchorage and retentive effects of a bisphosphonate (AHBuBP) on tooth movements in rats. Am J Orthod Dentofacial Orthop. 1994. 106:279–289.
Article20. Clark WG, Brater DC, Johnson AR. Nonsteroidal anti-inflammatory antipyretic analgesics. Goth's Medical Pharmacology. 1992. 13th ed. St Louis: Mosby-Year Book;356–373.21. Han JW, Kim SH. Effects of bisphosphonates on developing hard tissues of the jaws in rats. J Dent Science. 2001. 13:194–210.22. Agnusdei D, Bufalino L. Efficacy of ipriflavone in established osteoporosis and long-term safety. Calcif Tissue Int. 1997. 61:Suppl 1. S23–S27.
Article23. Kovács AB. Efficacy of ipriflavone in the prevention and treatment of postmenopausal osteoporosis. Agents Actions. 1994. 41:86–87.
Article24. Reginster JY. Ipriflavone: pharmacological properties and usefulness in postmenopausal osteoporosis. Bone Miner. 1993. 23:223–232.
Article25. Gennari C. Ipriflavone: background. Calcif Tissue Int. 1997. 61:Suppl 1. S3–S4.
Article26. Civitelli R. In vitro and in vivo effects of ipriflavone on bone formation and bone biomechanics. Calcif Tissue Int. 1997. 61:Suppl 1. S12–S14.
Article27. Martini M, Formigli L, Tonelli P, Giannelli M, Amunni F, Naldi D, et al. Effects of ipriflavone on perialveolar bone formation. Calcif Tissue Int. 1998. 63:312–319.
Article28. Notoya K, Tsukuda R, Yoshida K, Taketomi S. Stimulatory effect of ipriflavone on formation of bone-like tissue in rat bone marrow stromal cell culture. Calcif Tissue Int. 1992. 51:Suppl 1. S16–S20.
Article29. Notoya K, Yoshida K, Tsukuda R, Taketomi S. Effect of ipriflavone on expression of markers characteristic of the osteoblast phenotype in rat bone marrow stromal cell culture. J Bone Miner Res. 1994. 9:395–400.
Article30. Morita I, Sakaguchi K, Kurachi T, Murota S. Ipriflavone inhibits murine osteoclast formation in vitro. Calcif Tissue Int. 1992. 51:Suppl 1. S7–S10.31. Benvenuti S, Petilli M, Frediani U, Tanini A, Fiorelli G, Bianchi S, et al. Binding and bioeffects of ipriflavone on a human preosteoclastic cell line. Biochem Biophys Res Commun. 1994. 201:1084–1089.
Article32. Waldo CM, Rothblatt JM. Histologic response to tooth movement in the laboratory rat; procedure and preliminary observations. J Dent Res. 1954. 33:481–486.
Article33. Yokoya K, Sasaki T, Shibasaki Y. Distributional changes of osteoclasts and pre-osteoclastic cells in periodontal tissues during experimental tooth movement as revealed by quantitative immunohistochemistry of H(+)-ATPase. J Dent Res. 1997. 76:580–587.
Article34. Civitelli R, Abbasi-Jarhomi SH, Halstead LR, Dimarogonas A. Ipriflavone improves bone density and biomechanical properties of adult male rat bones. Calcif Tissue Int. 1995. 56:215–219.
Article35. Cecchini MG, Fleisch H, Mühibauer RC. Ipriflavone inhibits bone resorption in intact and ovariectomized rats. Calcif Tissue Int. 1997. 61:Suppl 1. S9–S11.
Article36. Perugini P, Genta I, Conti B, Modena T, Pavanetto F. Periodontal delivery of ipriflavone: new chitosan/PLGA film delivery system for a lipophilic drug. Int J Pharm. 2003. 252:1–9.
Article37. Bonucci E, Ballanti P, Martelli A, Mereto E, Brambilla G, Bianco P, et al. Ipriflavone inhibits osteoclast differentiation in parathyroid transplanted parietal bone of rats. Calcif Tissue Int. 1992. 50:314–319.
Article38. Cheng SL, Zhang SF, Nelson TL, Warlow PM, Civitelli R. Stimulation of human osteoblast differentiation and function by ipriflavone and its metabolites. Calcif Tissue Int. 1994. 55:356–362.
Article39. Lee YS, Kim YJ, Lee KH, Hwang HS. Effects of ipriflavone on bone remodeling in the rat calvarial cell. Korean J Orthod. 2005. 35:275–285.40. Lindhe J, Karring T, Araujo M. Lindhe J, Lang NP, Karring T, editors. The anatomy of periodontal tissues. Clinical Periodontology and Implant Dentistry. 2008. 5th ed. Oxford: Blackwell;27–31.41. Maeder CL, Carnes DL, Graves DT. Alkaline phosphatase and osteocalcin levels in cells from periodontal explants. J Dent Res. 1988. 67:232. Abst. No. 958.42. Piche JE, Carnes DL Jr, Graves DT. Initial characterization of cells derived from human periodontia. J Dent Res. 1989. 68:761–767.
Article43. Roberts WE, Chase DC. Kinetics of cell proliferation and migration associated with orthodontically-induced osteogenesis. J Dent Res. 1981. 60:174–181.
Article44. Basdra EK, Komposch G. Transmission and scanning electron microscopic analysis of mineralized loci formed by human periodontal ligament cells in vitro. J Orofac Orthop. 1999. 60:77–86.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The change of tooth mobility following orthodontic tooth movement ; A short-term study
- The study on the periodontal vascular changes of rat incisors following experimental tooth movement
- A histological and histochemical study on the periodontal tissue reaction during experimental tooth movement in the rat
- The effects of transforming growth factor-beta on the viability of human periodontal ligament cell and on the experimental tooth movement in rat
- The Effect of Occlusion on the Reorganization of Periodontal Fibers during Retention Periods after Tooth Movement in Rats