Korean J Obstet Gynecol.
2010 Apr;53(4):303-312. 10.5468/kjog.2010.53.4.303.
The usefulness of Doppler ultrasonography and the perinatal outcome of fetal anemia treated with intraumbilical venous transfusion
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. hswon@amc.seoul.kr
- KMID: 2273857
- DOI: http://doi.org/10.5468/kjog.2010.53.4.303
Abstract
OBJECTIVE
We undertook this study to determine the clinical characteristics and the prognostic factors of neonatal survival in patients with fetal anemia who were treated by intraumbilical venous transfusion (IUT).
METHODS
From July 2000 to March 2009, 16 cases of fetal anemia were diagnosed at Asan Medical Center in Seoul, Korea. These patients underwent intraumbilical venous transfusions and were thus included in our study. Doppler measurement of the middle cerebral artery peak systolic velocity was performed before and after cordocentesis in all fetuses.
RESULTS
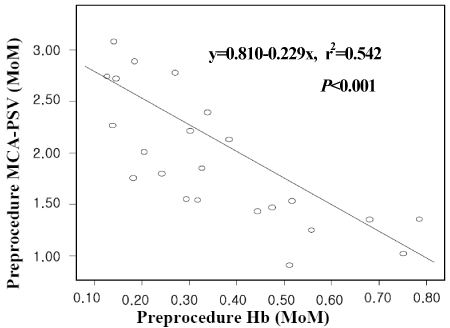
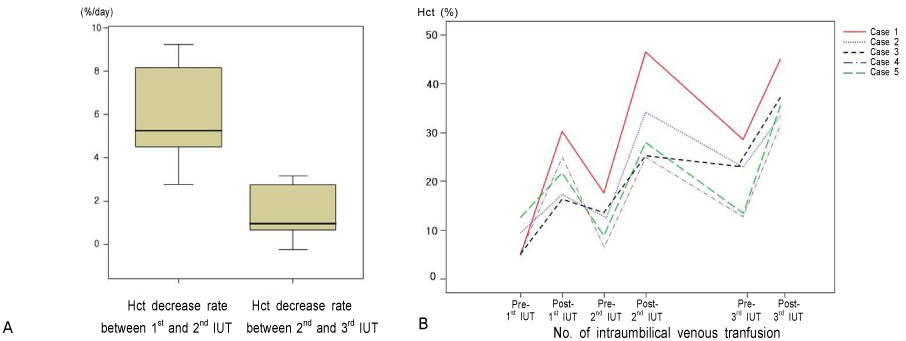
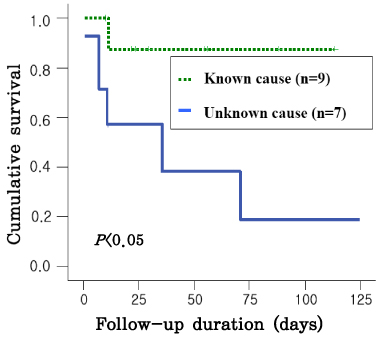
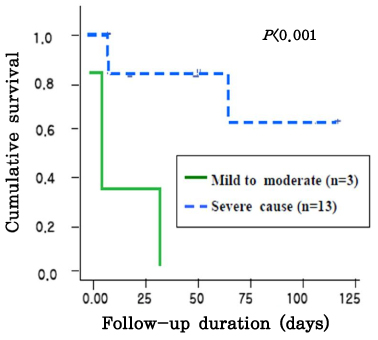
The gestational age at the time of the diagnosis of anemia ranged from 21.3 to 33.6 weeks. There was a linear correlation between pre- and post-procedure fetal hemoglobin (Hb,MoM, (x)) and the MCA-PSV (MoM, (y)), i.e., y=0.810-0.229x, r2=0.542, CI 0.316-0.141, p<0.005; and y=1.374-0.391x, r2=0.499, CI 0.584-0.197, p<0.005. The survival was better in patients with severe anemia than those with mild to moderate anemia (p<0.05), and survival was better in patients with anemia of a known cause than those with anemia of an unknown cause (p<0.001).
CONCLUSION
In fetuses with anemia, the severity of the anemia before IUT and the change of hemoglobin concentration after IUT, can be estimated noninvasively using Doppler ultrasonography, on the basis of an increase in the peak velocity of systolic blood flow in the middle cerebral artery. Both severity and etiology were meaningful factors for the survival of neonates with fetal anemia who were treated by intraumbilical venous transfusion. Although fetuses have severe anemia, they expected improved survival through IUT. These data are valuable information for use when counseling the parents of an affected fetus.
Keyword
MeSH Terms
Figure
Reference
-
1. Oberhoffer R, Grab D, Keckstein J, Hogel J, Terinde R, Lang D. Cardiac changes in fetuses secondary to immune hemolytic anemia and their relation to hemoglobin and catecholamine concentrations in fetal blood. Ultrasound Obstet Gynecol. 1999. 13:396–400.
Article2. Grannum PA, Copel JA, Moya FR, Scioscia AL, Robert JA, Winn HN, et al. The reversal of hydrops fetalis by intravascular intrauterine transfusion in severe isoimmune fetal anemia. Am J Obstet Gynecol. 1988. 158:914–919.
Article3. Machin GA. Hydrops revisited: literature review of 1,414 cases published in the 1980s. Am J Med Genet. 1989. 34:366–390.4. Nicolaides KH, Rodeck CH, Mibashan RS, Kemp JR. Have Liley charts outlived their usefulness? Am J Obstet Gynecol. 1986. 155:90–94.
Article5. Kaufman GE, Paidas MJ. Rhesus sensitization and alloimmune thrombocytopenia. Semin Perinatol. 1994. 18:333–349.6. Mari G, Deter RL, Carpenter RL, Rahman F, Zimmerman R, Moise KJ Jr, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med. 2000. 342:9–14.7. Weiner CP, Williamson RA, Wenstrom KD, Sipes SL, Widness JA, Grant SS, et al. Management of fetal hemolytic disease by cordocentesis. II. Outcome of treatment. Am J Obstet Gynecol. 1991. 165:1302–1307.8. Zimmerman R, Carpenter RJ Jr, Durig P, Mari G. Longitudinal measurement of peak systolic velocity in the fetal middle cerebral artery for monitoring pregnancies complicated by red cell alloimmunisation: a prospective multicentre trial with intention-to-treat. BJOG. 2002. 109:746–752.9. Oepkes D, Seaward PG, Vandenbussche FP, Windrim R, Kingdom J, Beyene J, et al. Doppler ultrasonography versus amniocentesis to predict fetal anemia. N Engl J Med. 2006. 355:156–164.
Article10. Scheier M, Hernandez-Andrade E, Fonseca EB, Nicolaides KH. Prediction of severe fetal anemia in red blood cell alloimmunization after previous intrauterine transfusions. Am J Obstet Gynecol. 2006. 195:1550–1556.
Article11. van Dongen H, Klumper FJ, Sikkel E, Vandenbussche FP, Oepkes D. Non-invasive tests to predict fetal anemia in Kell-alloimmunized pregnancies. Ultrasound Obstet Gynecol. 2005. 25:341–345.
Article12. Detti L, Mari G. Noninvasive diagnosis of fetal anemia. Clin Obstet Gynecol. 2003. 46:923–930.
Article13. Teixeira JM, Duncan K, Letsky E, Fisk NM. Middle cerebral artery peak systolic velocity in the prediction of fetal anemia. Ultrasound Obstet Gynecol. 2000. 15:205–208.
Article14. Fan FC, Chen RY, Schuessler GB, Chien S. Effects of hematocrit variations on regional hemodynamics and oxygen transport in the dog. Am J Physiol. 1980. 238:H545–H522.
Article15. Leung WC, Oepkes D, Seaward G, Ryan G. Serial sonographic findings of four fetuses with homozygous alpha-thalassemia-1 from 21 weeks onwards. Ultrasound Obstet Gynecol. 2002. 19:56–59.
Article16. Egberts J, van Kamp IL, Kanhai HH, Meerman RH, Giordano PC, Gravenhorst JB. The disappearance of fetal and donor red blood cells in alloimmunised pregnancies: a reappraisal. Br J Obstet Gynaecol. 1997. 104:818–824.
Article17. Radunovic N, Lockwood CJ, Alvarez M, Plecas D, Chitkara U, Berkowitz RL. The severely anemic and hydropic isoimmune fetus: changes in fetal hematocrit associated with intrauterine death. Obstet Gynecol. 1992. 79:390–393.18. Vaughan JI, Warwick R, Letsky E, Nicolini U, Rodeck CH, Fisk NM. Erythropoietic suppression in fetal anemia because of Kell alloimmunization. Am J Obstet Gynecol. 1994. 171:247–252.
Article19. Van Kamp IL, Klumper FJ, Oepkes D, Meerman RH, Scherjon SA, Vandenbussche FP, et al. Complications of intrauterine intravascular transfusion for fetal anemia due to maternal red-cell alloimmunization. Am J Obstet Gynecol. 2005. 192:171–177.
Article20. Chavez GF, Mulinare J, Edmonds LD. Epidemiology of Rh hemolytic disease of the newborn in the United States. JAMA. 1991. 265:3270–3274.
Article21. Schwarz TF, Nerlich A, Hottentrager B, Jager G, Wiest I, Kantimm S, et al. Parvovirus B19 infection of the fetus. Histology and in situ hybridization. Am J Clin Pathol. 1991. 96:121–126.
Article22. Wright C, Hinchliffe SA, Taylor C. Fetal pathology in intrauterine death due to parvovirus B19 infection. Br J Obstet Gynaecol. 1996. 103:133–136.
Article23. Public Health Laboratory Service Working Party on Fifth Disease. Prospective study of human parvovirus (B19) infection in pregnancy. BMJ. 1990. 300:1166–1170.24. Graves GR, Baskett TF. Nonimmune hydrops fetalis: antenatal diagnosis and management. Am J Obstet Gynecol. 1984. 148:563–565.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Fetal Erythroblastosis Treated by Repeated Intraumbilical Venous Transfusions
- A Case of Multiple Placental Chorioangioma Combined with Oligohydramnios
- Two cases of massive fetomaternal hemorrhage treated by exchange transfusion
- Color Doppler Ultrasound in Chronic Venous Insufficiency
- Neonatal Severe Anemia Due to Massive Fetomaternal Hemorrhage