Ann Rehabil Med.
2015 Apr;39(2):155-162. 10.5535/arm.2015.39.2.155.
Aging of Skeletal Muscle Fibers
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, Vanderbilt University School of Medicine, Nashville, TN, USA. walter.frontera@vanderbilt.edu
- 2Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
- 4Department of Physiology, University of Puerto Rico School of Medicine, San Juan, Puerto Rico.
- KMID: 2273035
- DOI: http://doi.org/10.5535/arm.2015.39.2.155
Abstract
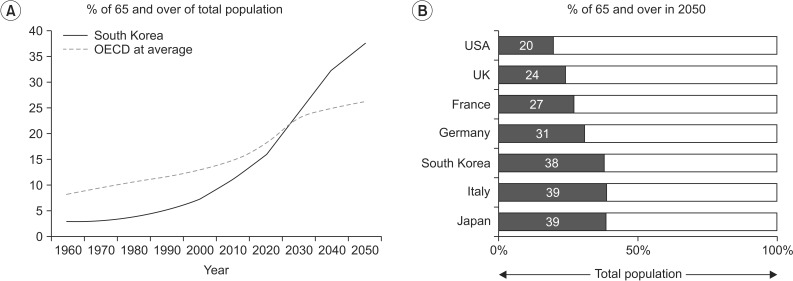
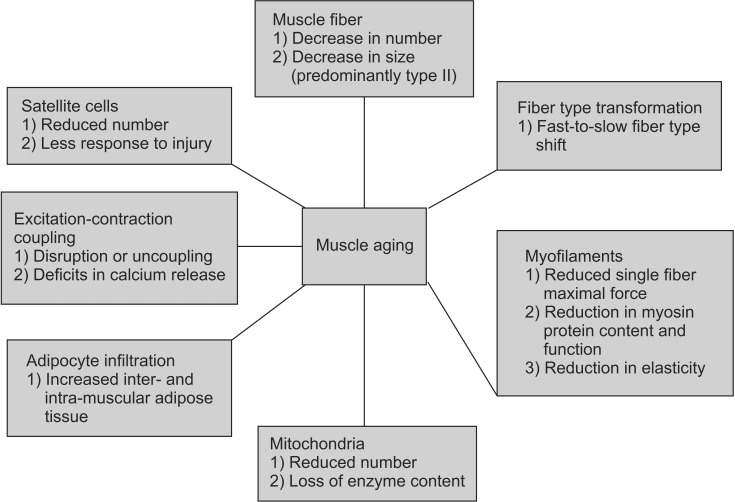
- Aging has become an important topic for scientific research because life expectancy and the number of men and women in older age groups have increased dramatically in the last century. This is true in most countries of the world including the Republic of Korea and the United States. From a rehabilitation perspective, the most important associated issue is a progressive decline in functional capacity and independence. Sarcopenia is partly responsible for this decline. Many changes underlying the loss of muscle mass and force-generating capacity of skeletal muscle can be understood at the cellular and molecular levels. Muscle size and architecture are both altered with advanced adult age. Further, changes in myofibers include impairments in several physiological domains including muscle fiber activation, excitation-contraction coupling, actin-myosin cross-bridge interaction, energy production, and repair and regeneration. A thorough understanding of these alterations can lead to the design of improved preventative and rehabilitative interventions, such as personalized exercise training programs.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Handgrip Strength: An Irreplaceable Indicator of Muscle Function
Sang Yoon Lee
Ann Rehabil Med. 2021;45(3):167-169. doi: 10.5535/arm.21106.Quality Matters as Much as Quantity of Skeletal Muscle: Clinical Implications of Myosteatosis in Cardiometabolic Health
Hong-Kyu Kim, Chul-Hee Kim
Endocrinol Metab. 2021;36(6):1161-1174. doi: 10.3803/EnM.2021.1348.
Reference
-
1. World Health Organization [Internet]. Geneva: World Health Organization;c2015. cited 2015 Feb 16. Available from: http://www.who.int/.2. Countryeconomy.com [Internet]. [place unknown]: Countryeconomy.com;c2015. cited 2015 Feb 16. Available from: http://countryeconomy.com/.3. Brady AO, Straight CR, Evans EM. Body composition, muscle capacity, and physical function in older adults: an integrated conceptual model. J Aging Phys Act. 2014; 22:441–452. PMID: 23945551.
Article4. Gill TM, Allore HG, Hardy SE, Guo Z. The dynamic nature of mobility disability in older persons. J Am Geriatr Soc. 2006; 54:248–254. PMID: 16460375.
Article5. Gill TM, Kurland B. The burden and patterns of disability in activities of daily living among community-living older persons. J Gerontol A Biol Sci Med Sci. 2003; 58:70–75. PMID: 12560415.
Article6. Hardy SE, Dubin JA, Holford TR, Gill TM. Transitions between states of disability and independence among older persons. Am J Epidemiol. 2005; 161:575–584. PMID: 15746474.
Article7. Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004; 291:1596–1602. PMID: 15069047.
Article8. Manini TM, Visser M, Won-Park S, Patel KV, Strotmeyer ES, Chen H, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007; 55:451–457. PMID: 17341251.
Article9. Manini T. Development of physical disability in older adults. Curr Aging Sci. 2011; 4:184–191. PMID: 21529321.
Article10. Rosenberg IH. Summary comments. Am J Clin Nutr. 1989; 50:1231–1233.
Article11. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997; 127(5 Suppl):990S–991S. PMID: 9164280.
Article12. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010; 39:412–423. PMID: 20392703.
Article13. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014; 69:547–558. PMID: 24737557.
Article14. Ryu M, Jo J, Lee Y, Chung YS, Kim KM, Baek WC. Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: the Fourth Korea National Health and Nutrition Examination Survey. Age Ageing. 2013; 42:734–740. PMID: 23761456.
Article15. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000; 88:1321–1326. PMID: 10749826.
Article16. Yamada M, Moriguch Y, Mitani T, Aoyama T, Arai H. Age-dependent changes in skeletal muscle mass and visceral fat area in Japanese adults from 40 to 79 years-of-age. Geriatr Gerontol Int. 2014; 14(Suppl 1):8–14. PMID: 24450556.
Article17. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001; 56:B209–B217. PMID: 11320101.
Article18. Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, et al. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014; 114:29–39. PMID: 24122149.
Article19. Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013; 14:329–340. PMID: 23698583.
Article20. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013; 93:23–67. PMID: 23303905.
Article21. Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006; 294:50–66. PMID: 16554047.
Article22. Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007; 292:E151–E157. PMID: 16926381.
Article23. McKay BR, Ogborn DI, Baker JM, Toth KG, Tarnopolsky MA, Parise G. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am J Physiol Cell Physiol. 2013; 304:C717–C728. PMID: 23392112.
Article24. Dumitru D, Amato AA, Zwarts MJ. Electrodiagnostic medicine. 2nd ed. Philadelphia: Hanley & Belfus Inc.;2002.25. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995; 50 Spec No:11–16. PMID: 7493202.26. Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002; 282:R519–R527. PMID: 11792662.27. Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol. 2000; 279:C611–C618. PMID: 10942711.
Article28. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000; 89:81–88. PMID: 10904038.
Article29. Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci. 2011; 4:192–199. PMID: 21529329.30. Delbono O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr Aging Sci. 2011; 4:248–259. PMID: 21529320.
Article31. Kostek MC, Delmonico MJ. Age-related changes in adult muscle morphology. Curr Aging Sci. 2011; 4:221–233. PMID: 21529325.32. D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, et al. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003; 552(Pt 2):499–511. PMID: 14561832.33. Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007; 62:375–381. PMID: 17452730.
Article34. Russ DW, Grandy JS, Toma K, Ward CW. Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiol (Oxf). 2011; 201:391–403. PMID: 20874807.
Article35. Yu F, Hedstrom M, Cristea A, Dalen N, Larsson L. Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol (Oxf). 2007; 190:229–241. PMID: 17581136.
Article36. D'Antona G, Pellegrino MA, Carlizzi CN, Bottinelli R. Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol. 2007; 100:603–611. PMID: 17273882.37. Delbono O. Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J Nutr Health Aging. 2000; 4:162–164. PMID: 10936903.38. Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J Membr Biol. 1997; 157:247–253. PMID: 9178612.
Article39. Wang Y, Pessin JE. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care. 2013; 16:243–250. PMID: 23493017.
Article40. Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013; 45:2191–2199. PMID: 23702032.
Article41. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012; 67:28–40. PMID: 21444359.
Article42. Anderson AA, Altafaj X, Zheng Z, Wang ZM, Delbono O, Ronjat M, et al. The junctional SR protein JP-45 affects the functional expression of the voltage-dependent Ca2+ channel Cav1.1. J Cell Sci. 2006; 119(Pt 10):2145–2155. PMID: 16638807.
Article43. Miller MS, Toth MJ. Myofilament protein alterations promote physical disability in aging and disease. Exerc Sport Sci Rev. 2013; 41:93–99. PMID: 23392279.
Article44. Moen RJ, Klein JC, Thomas DD. Electron paramagnetic resonance resolves effects of oxidative stress on muscle proteins. Exerc Sport Sci Rev. 2014; 42:30–36. PMID: 24188980.
Article45. Lowe DA, Thomas DD, Thompson LV. Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. Am J Physiol Cell Physiol. 2002; 283:C187–C192. PMID: 12055087.46. Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, et al. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol (1985). 2008; 105:637–642. PMID: 18556434.
Article47. Reid KF, Doros G, Clark DJ, Patten C, Carabello RJ, Cloutier GJ, et al. Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol. 2012; 112:2289–2301. PMID: 22005960.
Article48. Monroy JA, Powers KL, Gilmore LA, Uyeno TA, Lindstedt SL, Nishikawa KC. What is the role of titin in active muscle. Exerc Sport Sci Rev. 2012; 40:73–78. PMID: 22157359.
Article49. Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985). 2001; 90:2157–2165. PMID: 11356778.
Article50. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000; 89:104–110. PMID: 10904041.
Article51. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009; 90:1579–1585. PMID: 19864405.52. Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002; 166:479–484. PMID: 12186824.53. Broskey NT, Greggio C, Boss A, Boutant M, Dwyer A, Schlueter L, et al. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014; 99:1852–1861. PMID: 24438376.
Article54. Hepple RT. Mitochondrial involvement and impact in aging skeletal muscle. Front Aging Neurosci. 2014; 6:211. PMID: 25309422.
Article55. Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008; 43:24–33. PMID: 17997255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The changes of the skeletal muscle fiber type after cross innervation in the rat I. histochemical stain & immunohistochemical stain
- In Vivo Rodent Models of Skeletal Muscle Adaptation to Decreased Use
- The studies of the skeletal muscle fiber after cross innervation in the rat: II>morphometric studies on the ultrastructure using electron microscopy
- Experimental Studies on Disuse Atrophy of the Rat Tibialis Anterior Muscle
- Morphological Studies on the Effects of Lidocaine and Tetracaine on Rat Gracilis Muscle