Blood Res.
2014 Dec;49(4):234-240. 10.5045/br.2014.49.4.234.
Monosomal and complex karyotypes as prognostic parameters in patients with International Prognostic Scoring System higher risk myelodysplastic syndrome treated with azacitidine
- Affiliations
-
- 1Department of Hematology-Oncology, Pusan National University Hospital, Busan, Korea. Hemon@pusan.ac.kr
- 2Department of Hematology, Kyungpook National University Hospital, Daegu, Korea.
- 3Department of Hematology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 4Department of Hematology, Chungnam Nastional Ujnversity Hospital, Daejeon, Korea.
- 5Department of Hematology, Gachon University Gil Medical Center, Incheon, Korea.
- 6Department of Hematology, Gyeong-Sang National University Hospital, School of Medicine, Gyeongsang Natioinal University, Jinju, Korea.
- KMID: 2270680
- DOI: http://doi.org/10.5045/br.2014.49.4.234
Abstract
- BACKGROUND
Azacitidine (AZA) is standard care for patients with myelodysplastic syndrome (MDS) who have not had allogeneic stem cell transplantation. Chromosomal abnormalities (CA) including complex karyotype (CK) or monosomal karyotype (MK) are associated with clinical outcome in patients with MDS.
METHODS
We investigated which prognostic factors including CAs would predict clinical outcomes in patients with International Prognostic Scoring System (IPSS) higher risk MDS treated with AZA, retrospectively. CK was defined as the presence of three or more numerical or structural CAs. MK was defined as the presence of two or more distinct autosomal monosomies or single autosomal monosomy with at least one additional structural CA.
RESULTS
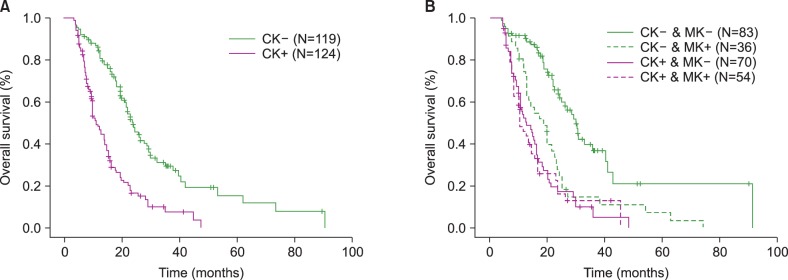
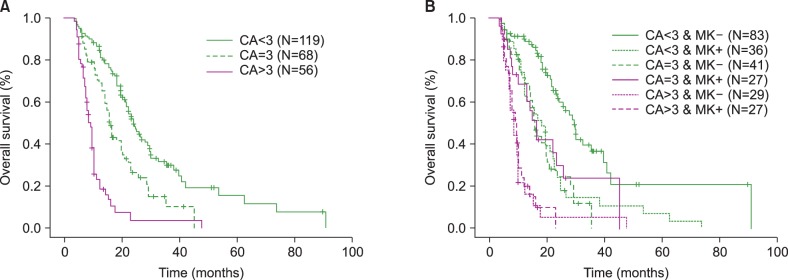
A total of 243 patients who treated with AZA, were enrolled. CK was present in 124 patients and MK was present in 90 patients. Bone marrow blasts > or =15% and CK were associated with poorer response (P=0.038, P=0.007) and overall survival (OS) (P<0.001, P<0.001) independently. Although MK in CK group was not associated with prognosis, non-MK status in non-CK group reflected favorable OS (P=0.005). The group including >3 CAs was associated with poorer OS (group including <3 CAs vs. only three CAs, P=0.001; group with >3 CAs vs. only three CAs, P=0.001).
CONCLUSION
CK was an important prognostic parameter associated with worse outcome. MK may predict poor survival in only non-CK status. The higher number of CAs was associated with poorer survival.
Keyword
MeSH Terms
Figure
Reference
-
1. Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009; 361:1872–1885. PMID: 19890130.
Article2. Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997; 89:2079–2088. PMID: 9058730.
Article3. Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002; 20:2429–2440. PMID: 12011120.
Article4. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010; 28:562–569. PMID: 20026804.
Article5. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009; 10:223–232. PMID: 19230772.
Article6. Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007; 25:3503–3510. PMID: 17687155.
Article7. Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007; 110:4385–4395. PMID: 17726160.
Article8. Schanz J, Steidl C, Fonatsch C, et al. Coalesced multicentric analysis of 2,351 patients with myelodysplastic syndromes indicates an underestimation of poor-risk cytogenetics of myelodysplastic syndromes in the international prognostic scoring system. J Clin Oncol. 2011; 29:1963–1970. PMID: 21519021.
Article9. Breems DA, Van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008; 26:4791–4797. PMID: 18695255.
Article10. Patnaik MM, Hanson CA, Hodnefield JM, Knudson R, Van Dyke DL, Tefferi A. Monosomal karyotype in myelodysplastic syndromes, with or without monosomy 7 or 5, is prognostically worse than an otherwise complex karyotype. Leukemia. 2011; 25:266–270. PMID: 21072042.
Article11. Belli CB, Bengio R, Aranguren PN, et al. Partial and total monosomal karyotypes in myelodysplastic syndromes: comparative prognostic relevance among 421 patients. Am J Hematol. 2011; 86:540–545. PMID: 21674572.
Article12. Itzykson R, Thepot S, Eclache V, et al. Prognostic significance of monosomal karyotype in higher risk myelodysplastic syndrome treated with azacitidine. Leukemia. 2011; 25:1207–1209. PMID: 21468040.
Article13. Shaffer LG, McGowan-Jordan J, Schmid M. ISCN 2013. An international system for human cytogenetic nomenclature. Recommendations of the International Standing Committee on Human Cytogenetic Nomenclature. Basel, Switzerland: Karger;2013.14. Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006; 108:419–425. PMID: 16609072.
Article15. Wijermans PW, Lubbert M, Verhoef G, Klimek V, Bosly A. An epigenetic approach to the treatment of advanced MDS; the experience with the DNA demethylating agent 5-aza-2'-deoxycytidine (decitabine) in 177 patients. Ann Hematol. 2005; 84(Suppl 1):9–17. PMID: 16211386.
Article16. Kantarjian HM, O'Brien S, Shan J, et al. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007; 109:265–273. PMID: 17133405.
Article17. Itzykson R, Thepot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011; 117:403–411. PMID: 20940414.
Article18. Valcarcel D, Adema V, Sole F, et al. Complex, not monosomal, karyotype is the cytogenetic marker of poorest prognosis in patients with primary myelodysplastic syndrome. J Clin Oncol. 2013; 31:916–922. PMID: 23319689.19. Schanz J, Tuchler H, Sole F, et al. Monosomal karyotype in MDS: explaining the poor prognosis? Leukemia. 2013; 27:1988–1995. PMID: 23787396.
Article20. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012; 120:2454–2465. PMID: 22740453.21. Bernasconi P, Boni M, Cavigliano PM, et al. Clinical relevance of cytogenetics in myelodysplastic syndromes. Ann N Y Acad Sci. 2006; 1089:395–410. PMID: 17261783.
Article22. Boultwood J, Lewis S, Wainscoat JS. The 5q-syndrome. Blood. 1994; 84:3253–3260. PMID: 7949083.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Classifications and prognostic scoring systems in myelodysplastic syndrome
- Diagnosis and Treatment of Myelodysplastic Syndrome in the Era of Genetic Testing
- Azacitidine-Induced Lung Injury in a Patient with Myelodysplastic Syndrome
- A Clinical Study of Pediatric Myelodysplastic Syndrome: Application of International Prognostic Scoring System and the Review of the Korean Literature
- Azacitidine-induced Hepatotoxicity in a Patient with Myelodysplastic Syndrome