Asian Oncol Nurs.
2012 Sep;12(3):195-203. 10.5388/aon.2012.12.3.195.
Effectiveness of Physical Exercise in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation
- Affiliations
-
- 1Yonsei University College of Nursing, Gangneung, Korea. sujin@gyc.ac.kr
- 2Chung-Ang University Red Cross College of Nursing, Seoul, Korea.
- KMID: 2269505
- DOI: http://doi.org/10.5388/aon.2012.12.3.195
Abstract
- PURPOSE
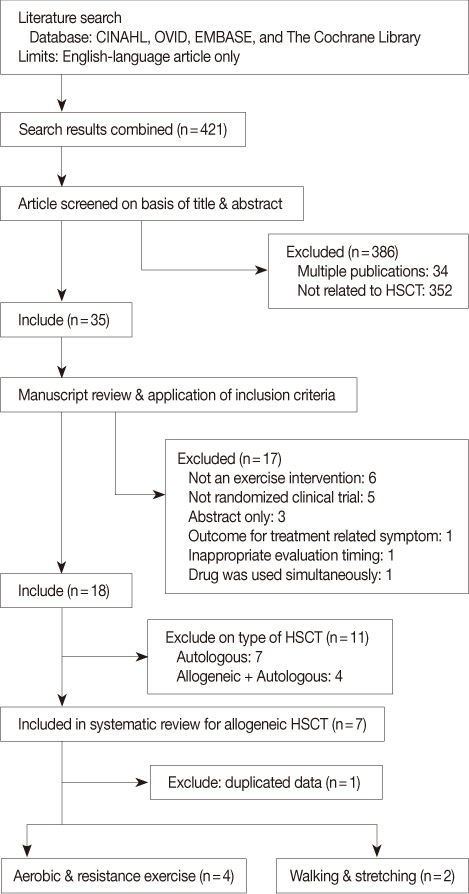
To summarize and review the methodological quality of the evidence from trials examining the effectiveness of physical exercise in patients undergoing allogeneic hematopoietic stem cell transplantation (Allo-HSCT).
METHODS
Six randomized clinical trials (RCTs) were identified, reviewed for substantive results, and assessed for methodological quality.
RESULTS
Six trials met all methodological criteria on the modified Jadad score above 3 out of 5 points. Failure to blind the outcome assessor, and failure to describe the method of blinding of outcome assessor appropriately were the most prevalent methodological shortcomings. Various exercise modalities have been applied, differing in content, frequency, intensity, and duration. Positive results have been observed in part for a diverse set of outcomes, including physical and psychological performance.
CONCLUSION
The trials reviewed in this study were of moderate methodological quality. They suggest that exercise in patients undergoing Allo-HSCT may be safe and feasible, and in part patients benefit from increased physical performance both during and after transplantation. Future RCTs should use larger samples, appropriate comparison groups, and a standard of outcome measures, and examine what kind of exercise intervention (aerobic vs. resistance vs. combined) is the most effective for Allo-HSCT patients. It would be necessary to define contraindication for exercise to guarantee its safety.
MeSH Terms
Figure
Reference
-
1. National Cancer Information Center. accessed on 30 April 2011. Available at: http://www.cancer.go.kr/cms/publicproject/education/index.html.2. Korean National Statistical Office. Statistics for the Cause of Death on 2012.3. Park HJ, Park EH, Jung KW, Kong HJ, Won YJ, Lee JY, et al. Statistics of hematologic malignancies in Korea: Incidence, prevalence and survival rates from 1999 to 2008. Korean J Hematol. 2012. 47:28–38.4. Santos GW. Bone marrow transplantation in hematologic malignancies: Current status. Cancer. 1990. 65:786–791.
Article5. Kim MH. Main symptoms of cancer patients by stage in a general hospital [dissertation]. 2010. Seoul: Hanyang Univ..6. Kim KS, Cho MY. Hematopoietic stem cell transplantation experiences in Korea. Proceedings of the 9th Annual Meeting of the Korean Society of Hematopoietic Stem Cell Transplantation. 2004. 2004 Aug 20-21; Busan, Korea. Seoul: Medrang.7. Pallera AM, Schwartzberg LS. Managing the toxicity of hematopoietic stem cell transplant. J Support Oncol. 2004. 2:223.8. Courneya KS, Keats MR, Turner AR. Physical exercise and quality of life in cancer patients following high dose chemotherapy and autologous bone marrow transplantation. Psychooncology. 2000. 9:127–136.
Article9. Danaher EH, Ferrans C, Verlen E, Ravandi F, van Besien K, Gelms J, et al. Fatigue and physical activity in patients undergoing hematopoietic stem cell transplant. Oncol Nurs Forum. 2006. 33:614–624.
Article10. Schule K. The rank value of sports and movement therapy in patients with breast or pelvic cancer. Rehabilitation. 1983. 22:36–39.11. Cunningham BA, Morris G, Cheney CL, Buergel N, Aker SN, Lenssen P. Effects of resistive exercise on skeletal muscle in marrow transplant recipients receiving total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1986. 10:558–563.
Article12. Mello M, Tanaka C, Dulley FL. Effects of an exercise program on muscle performance in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant. 2003. 32:723–728.
Article13. Jadad AR, Moore RA, Carrol D, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials. 1996. 17:1–12.
Article14. Jarden M, Baadsgaard MT, Hovgaard DJ, Boesen E, Adamsen L. A randomized trial on the effect of a multimodal intervention on physical capacity, functional performance and quality of life in adult patients undergoing allogeneic SCT. Bone Marrow Transplant. 2009. 43:725–737.
Article15. Wiskemann J, Dreger P, Schwerdtfeger R, Bondong A, Huber G, Kleindienst N, et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood. 2011. 117:2604–2613.
Article16. Shelton ML, Lee JQ, Morris GS, Massey PR, Kendall DG, Munsell MF, et al. A randomized control trial of a supervised versus a self-directed exercise program for allogeneic stemcell transplant patients. Psycho-Oncology. 2009. 18:353–359.
Article17. San Juan AF, Chamorro-Viña C, Moral S, Fernández del Valle M, Madero L, Ramírez M, et al. Benefits of intrahospital exercise training after pediatric bone marrow transplantation. Int J Sports Med. 2008. 29:439–446.
Article18. DeFor TE, Burns LJ, Gold EM, Weisdorf DJ. A Randomized trial of the effect of a walking regimen on the functional status of 100 adult allogeneic donor hematopoietic cell transplant patients. Biol Blood Marrow Transplant. 2007. 13:948–955.
Article19. Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of random ised controlled trials. Clin Oncol (R Coll Radiol). 2010. 22:208–221.
Article20. Decker WA, Turner-McGlade J, Fehir KM. Psychosocial aspects and the physiological effects of a cardiopulmonary exercise program in patients undergoing bone marrow transplantation for acute leukemia. Transplant Proc. 1989. 21:3068–3069.21. Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005. 23:3830–3842.
Article22. Hayes SC, Spence RR, Galvao DA, Newton RU. Australian association for exercise and sport science position stand: optimising cancer outcomes through exercise. J Sci Med Sport. 2009. 12:428–434.
Article23. Szeluga DJ, Stuart RK, Brookmeyer R, Utermohlen V, Santos GW. Nutritional support of bone marrow parenteral nutrition to an enteral feeding program. Cancer Res. 1987. 47:3309–3316.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Quality of Life of Autologous and Allogeneic Hematopoietic Stem Cell Transplantation Recipients
- Effects of Back Massage on Immune Response, Symptom Distress and Mood State of Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation
- Pre- and Post-Transplant Nutritional Assessment in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation
- A case of pneumomediastinum combined with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation
- Allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes