Korean J Nutr.
2010 Dec;43(6):653-660. 10.4163/kjn.2010.43.6.653.
Human Studies on Functional Foods: How They Are Regulated
- Affiliations
-
- 1Department of Food & Nutritional Sciences, Ewha Womans University, Seoul 120-750, Korea. orank@ewha.ac.kr
- KMID: 2268061
- DOI: http://doi.org/10.4163/kjn.2010.43.6.653
Abstract
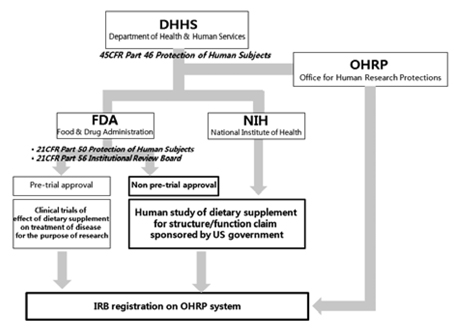
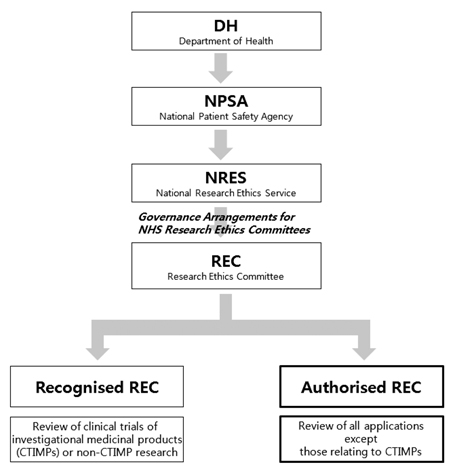
- Along with the steady growth of health functional food (HFF) markets, research evaluating the human effects of HFF has been expanding. In this study, we investigated the regulatory and management system of human study on HFF in the USA, Japan and UK, and the Korean domestic regulations on HHF, medicines, medical devices, cosmetics and biotechnology in order to improve the domestic management system. In these four countries, institutional review board (IRB) or research ethics committee (REC) approvals are required for on human study of HHF, but regulatory and management systems differ from country to country. In the USA, human studies on HFF for structure/function claims do not require the FDA's prior approval but clinical trials of the disease treatment effects of HHF require prior approval from the FDA. In the USA, IRBs are managed by the Department of Health and Human Services (DHHS) rather than the FDA, and IRBs in those institutions which would execute the clinical trials requiring prior approval from the FDA or human studies funded by the USA federal government are required to be registered on the DHHS. In the UK, although the government does not require prior approval of human study, authorized RECs managed by the National Research Ethics Service (NRES) and other independent RECs review the human study. In Japan, human study for HFF must conform with "Ethical guidelines for epidemiological research" and IRB registration has not been required. In Korean domestic regulations, the responsibilities, compositions, functions and operations of IRBs on medicines, medical devices and biotechnology are legally specified, but not those of IRB on HHF. These foreign statuses for the management of human study on HFF and comparisons with Korean regulations are expected to be used as basic data to improve the domestic legal system.
Keyword
MeSH Terms
Figure
Reference
-
1. Korean Ministry of Health & Welfare. Health Functional Food Acts. 2010.2. Korea Food & Drug Administration. White Paper for Food & Drug Safety, Korea Food & Drug Administration (Publication Registration No. 1-147000-000139-10). 2009.3. Korea Food & Drug Administration. Regulation on Approval of Functional Ingredient for Health Functional Food, Food and Drug Administration Notification No. 2010-76. 2010.4. Kim MK, Kwon OR, Chun HS, Won HS, Kim JY, Kang BC, Che JW, Han JG, Hong SW, Kim WS, Pee JH, Park HY, Kim HJ. Health Functional Food. 2010. Kyomunsa;29–31.5. Kim JS, Kwon OR. Health Functional Foods, What Do You Think about Them? Food Sci Ind. 2007. 40(2):3–10.6. Label Claims. US Food & Drug Administration. Available from: http://www.fda.gov/Food/LabelingNutrition/LabelClaims/default.htm.7. Guidance for Industry, A Food Labeling Guide. US Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Nutrition, Labeling and Dietary Supplements. 2008. Available from: http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodLabelingNutrition/FoodLabelingGu ide/default.htm.8. US Food & Drug Administration. 21 Code of Federal Regulations Part 50 Protection of Human Subjects. 2010. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?FR=50.9. 21 Code of Federal Regulaions Part 56 Institutional Review Boards. US Food & Drug Administration. 2010. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=56.10. 21 CFR 312 Investigational New Drug Application. U.S. Food & Drug Administration. 2010. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=312.11. Guidance for Industry, Botanical Drug Products. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). 2004. Available from: http://www.fda.gov/downloads/Drugs/Guidance-ComplianceRegulatoryInformation/Guidances/ucm070491.pdf.12. Frequently Asked Questions on Botanical Drug Product Development. US Food & Drug Administration. 2009. Available from: http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm090989.htm.13. 45 Code of Federal Regulation Part 46 Protection of Human Subjects. US Department of Health and Human Services. 2009. Available from: http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm.14. Registration of an Institutional Review Board. US Department of Health and Human Services, Office for Human Research Protections. 2009. Available from: http://www.hhs.gov/ohrp/assurances/.15. Food & Drug Administration Act, section 113. US Food & Drug Administration. 1997. Available from: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDAMA/FullTextofFDAMAlaw/default.htm.16. Kim MK, Kwon OR, Chun HS, Won HS, Kim JY, Kang BC, Che JW, Han JG, Hong SW, Kim WS, Pee JH, Park HY, Kim HJ. Health Functional Food. 2010. Kyomunsa;39–40.17. European Commision, Regulation (EC) No. 1924/2006. 2006. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:404:0009:0025:EN:PDF.18. Aggett PJ, Antoine JM, Asp NG, Bellisle F, Contor L, Cummings J, Howlett J, Muller D, Pijls L, Rechkemmer G, Tuijtelaars S, Verhagen H. PASSCLAIM, Consensus on Criteria. Eur J Nutr. 2005. 44:Suppl 1. i5–i30.19. European Food Safety Authority. Scientific and technical guidance for the preparation and presentation of the application for authorisation of a health claim (Request N° EFSA-Q-2007-066) (Adopted on 6 July 2007). EFSA Journal. 2007. 530:1–44.20. Korean Ministry of Education & National Research Foundation of Korea. Establishing and promoting research ethics: Cases of foreign universities. 2007. 23–24.21. UK Department of Health. Research Governance Frame work for Health and Social Care. 2005. Second Edition.22. About Research Ethics Commitee. UK. National Patient Safety Agency, National Research Ethics Service. Available from: http://www.nres.npsa.nhs.uk/.23. Governance arrangement for NHS Research Ethics Committees. UK Department of Health. 2001. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4005727.24. Kim MK, Kwon OR, Chun HS, Won HS, Kim JY, Kang BC, Che JW, Han JG, Hong SW, Kim WS, Pee JH, Park HY, Kim HJ. Health Functional Food. 2010. Kyomunsa;33–34.25. Japan Ministry of Health, Labour and Welfare. Notice on preparing the attached materials in applying for evaluation of FOSHU (Foods for Specified Health Uses). 2005.26. Ethical Guidelines for Epidemiological Research. Japan Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare. 2008. Available from: http://www.niph.go.jp/wadai/ekigakurinri/index.htm.27. Korean Ministry of Health & Welfare. Pharmaceutical Affairs Act. 2010.28. Korean Ministry of Health & Welfare. Medical Device Act. 2010.29. Korean Ministry of Health & Welfare. Cosmetic Act. 2010.30. Korean Ministry of Health & Welfare. Bioethics and Safety Act. 2010.31. Korean Ministry of Health & Welfare. Proposed revision of Bioethics and Safety Act. 2010.