Korean J Nutr.
2010 Aug;43(4):342-350. 10.4163/kjn.2010.43.4.342.
Effects of Soy Protein, its Hydrolysate and Peptide Fraction on Lipid Metabolism and Appetite-Related Hormones in Rats
- Affiliations
-
- 1Department of Food and Nutrition & Research Institute of Human Ecology, Seoul National University, Seoul 151-742, Korea. lysook@snu.ac.kr
- 2R & D Center, Maeil Dairies Co. Ltd., Pyungtaek 451-861, Korea.
- KMID: 2268022
- DOI: http://doi.org/10.4163/kjn.2010.43.4.342
Abstract
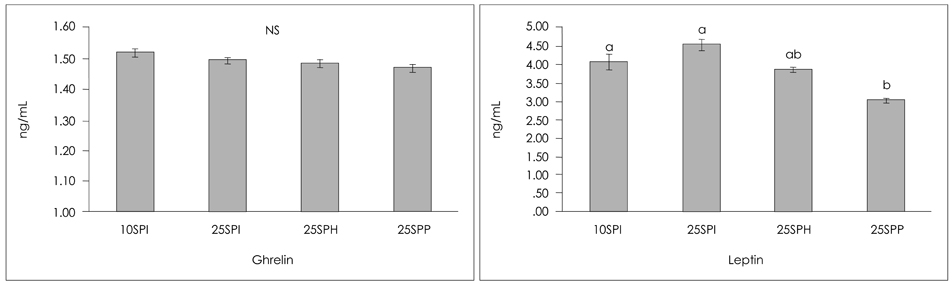
- This study was aimed to investigate whether soy protein hydrolysates had beneficial effects on serum and tissue lipid contents and appetite-related hormones as compared with intact soy protein. Four-week-old male Sprague-Dawley rats were fed AIN-93M diet containing high fat (18% w/w) with low protein (10% w/w). After four weeks, the rats were divided into four groups (n = 8/group) and fed experimental diets with different nitrogen sources and levels, respectively; 10% soy protein isolate (10SPI), 25% soy protein isolate (25SPI), 25% soy protein hydrolysates (25SPH) and 25% soy macro-peptide fractions (25SPP, MW > or = 10,000) for six weeks. Weight gain was significantly higher in 25% nitrogen sources-fed groups than in 10% group (10SPI). In 25SPP, perirenal fat mass and serum total lipid were significantly lower than in other groups. As for appetite-related hormones, serum ghrelin concentration was not shown to be different among groups but leptin concentration was significantly decreased in 25SPP. It can be concluded that soy macro-peptide fractions as compared with intact soy protein may have beneficial effects on reducing fat mass and serum lipid.
Keyword
MeSH Terms
Figure
Reference
-
1. Halton TL, Hu FB. The Effects of High Protein Diets on Thermogenesis, Satiety and Weight Loss: A Critical Review. J Am Coll Nutr. 2004. 23(5):373–385.
Article2. Alfenas Rde C, Bressan J, de Paiva AC. Effects of protein quality on appetite and energy metabolism in normal weight subjects. Arq Bras Endocrinol Metabol. 2010. 54(1):45–51.
Article3. Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Effects of high and normal soyprotein breakfasts on satiety and subsequent energy intake, including amino acid and 'satiety' hormone responses. Eur J Nutr. 2009. 48(2):92–100.
Article4. Baum JA, Teng H, Erdman JW Jr, Weigel RM, Klein BP, Persky VW, Freels S, Surya P, Bakhit RM, Ramos E, Shay NF, Potter SM. Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women. Am J Clin Nutr. 1998. 68(3):545–551.
Article5. Hurley C, Richard D, Deshaies Y, Jacques H. Soy protein isolate in the presence of cornstarch reduces body fat gain in rats. Can J Physiol Pharmacol. 1998. 76(10-11):1000–1007.
Article6. Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci. 2007. 4(2):72–82.
Article7. Aoyama T, Fukui K, Takamatsu K, Hashimoto Y, Yamamoto T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition. 2000. 16(5):349–354.
Article8. Torres N, Torre-Villalvazo I, Tovar AR. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. J Nutr Biochem. 2006. 17(6):365–373.
Article9. Noriega-López L, Tovar AR, Gonzalez-Granillo M, Hernández-Pando R, Escalante B, Santillán-Doherty P, Torres N. Pancreatic insulin secretion in rats fed a soy protein high fat diet depends on the interaction between the amino acid pattern and isoflavones. J Biol Chem. 2007. 282(28):20657–20666.
Article10. Aoyama T, Fukui K, Nakamori T, Hashimoto Y, Yamamoto T, Takamatsu K, Sugano M. Effect of soy and milk whey protein isolates and their hydrolysates on weight reduction in genetically obese mice. Biosci Biotechnol Biochem. 2000. 64(12):2594–2600.
Article11. Cho SJ, Juillerat MA, Lee CH. Cholesterol lowering mechanism of soybean protein hydrolysate. J Agric Food Chem. 2007. 55(26):10599–10604.
Article12. Sugano M, Goto S, Yamada Y, Yoshida K, Hashimoto Y, Matsuo T, Kimoto M. Cholesterol-lowering activity of various undigested fractions of soybean protein in rats. J Nutr. 1990. 120(9):977–985.
Article13. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123:1939–1951.
Article14. Frings CS, Dunn RM. A colorimetric method for determination of total serum lipids based on the sulfo-phospho-vanillin reaction. Am J Clin Pathol. 1970. 53:89–91.
Article15. Folch J, Less M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957. 226:497–502.16. Akahoshi A, Koba K, Enmoto R, Nishimura K, Honda Y, Minami M, Yamamoto K, Iwata T, Yamauchi Y, Tsutsumi K, Sugano M. Combined effects of dietary protein type and fat level on the body fat-reducing activity of conjugated linoleic acid (CLA) in rats. Biosci Biotechnol Biochem. 2005. 69(12):2409–2415.
Article17. Hammond KA, Janes DN. The effects of increased protein intake on kidney size and function. J Exp Biol. 1998. 201(Pt 13):2081–2090.
Article18. Murray BM, Campos SP, Schoenl M, MacGillivray MH. Effect of dietary protein intake on renal growth: possible role of insulin-like growth factor-I. J Lab Clin Med. 1993. 122(6):677–685.19. Gudbrandsen OA, Wergedahl H, Mørk S, Liaset B, Espe M, Berge RK. Dietary soya protein concentrate enriched with isoflavones reduced fatty liver increased hepatic fatty acid oxidation and decreased the hepatic mRNA level of VLDL receptor in obese Zucker rats. Br J Nutr. 2006. 96(2):249–257.
Article20. Uebanso T, Taketani Y, Fukaya M, Sato K, Takei Y, Sato T, Sawada N, Amo K, Harada N, Arai H, Yamamoto H, Takeda E. Hypocaloric high-protein diet improves fatty liver and hypertriglyceridemia in sucrose-fed obese rats via two pathways. Am J Physiol Endocrinol Metab. 2009. 297(1):E76–E84.
Article21. Liyanage R, Han KH, Watanabe S, Shimada K, Sekikawa M, Ohba K, Tokuji Y, Ohnishi M, Shibayama S, Nakamori T, Fukushima M. Potato and soy peptide diets modulate lipid metabolism in rats. Biosci Biotechnol Biochem. 2008. 72(4):943–950.
Article22. Hayashi S, Miyazaki Y, Yamashita J, Nakagawa M, Takizawa H. Soy protein has no hypocholesterolemic action in mice because it does not stimulate fecal steroid excretion in that species. Cell Mol Biol. 1994. 40(7):1021–1028.23. Claessens M, Saris WH, van Baak MA. Glucagon and insulin responses after ingestion of different amounts of intact and hydrolysed proteins. Br J Nutr. 2008. 100(1):61–69.
Article24. Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc. 2000. 59(3):359–371.
Article25. Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc Natl Acad Sci USA. 1996. 93(4):1726–1730.
Article26. Maurer AD, Chen Q, McPherson C, Reimer RA. Changes in satiety hormones and expression of genes involved in glucose and lipid metabolism in rats weaned onto diets high in fibre or protein reflect susceptibility to increased fat mass in adulthood. J Physiol. 2009. 587(Pt 3):679–691.
Article27. Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010. 55(4):862–868.
Article28. Gil-Campos M, Aguilera CM, Cañete R, Gil A. Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr. 2006. 96(2):201–226.
Article29. Dimaraki EV, Jaffe CA. Role of endogenous ghrelin in growth hormone secretion, appetite regulation and metabolism. Rev Endocr Metab Disord. 2006. 7(4):237–249.
Article30. Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000. 141(11):4325–4328.
Article31. Iqbal J, Kurose Y, Canny B, Clarke IJ. Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin, and cortisol secretion in sheep. Endocrinology. 2006. 147(1):510–519.
Article32. Lim CT, Kola B, Korbonits M, Grossman AB. Ghrelin's role as a major regulator of appetite and its other functions in neuroendocrinology. Prog Brain Res. 2010. 182:189–205.
Article33. Park JY, Park MN, Choi YY, Yun SS, Chun HN, Lee YS. Effects of whey protein hydrolysates on lipid profiles and appetite-related hormones in rats fed high fat diet. J Korean Soc Food Sci Nutr. 2008. 37(4):428–436.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity
- Effect of Genistein and Soy Protein on Lipids Metabolism in Ovariectomized Rats
- Effect of Soy Protein Hydrolyzate on Lipid Metabolism and Antioxidant Activity in the Rat
- Food Intake and Gut Hormones
- Effects of Soy and Isoflavones on Bone Metabolism in Growing Female Rats