Ann Dermatol.
2013 Feb;25(1):46-53. 10.5021/ad.2013.25.1.46.
Safety Evaluation of Stamp Type Digital Microneedle Devices in Hairless Mice
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Chung-Ang University, Seoul, Korea. beomjoon@unitel.co.kr
- 2Department of Life Science (BK21 program), College of Natural Sciences, Chung-Ang University, Seoul, Korea.
- 3Department of Emergency Medicine, College of Medicine, Chung-Ang University, Seoul, Korea.
- KMID: 2265970
- DOI: http://doi.org/10.5021/ad.2013.25.1.46
Abstract
- BACKGROUND
Microneedles provide a minimally invasive means to transport molecules into the skin. A number of specific strategies have been employed to use microneedles for transdermal delivery.
OBJECTIVE
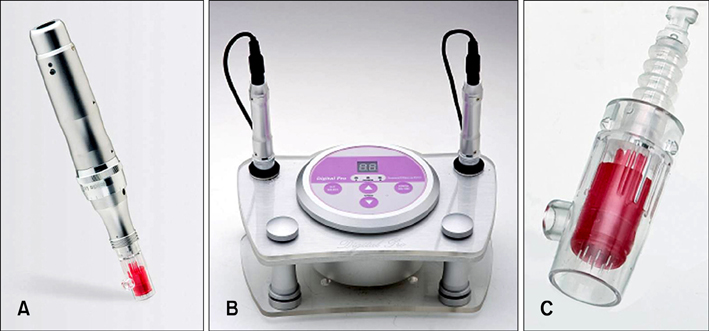
The purpose of this study was to investigate the safety of two new digital microneedle devices (Digital Hand(R) and Digital Pro(R); Bomtech Electronics Co., Ltd., Seoul, Korea) for the perforation of skin in skin-hairless-1 mice. This device replaces conventional needles and is designed specifically for intradermal delivery.
METHODS
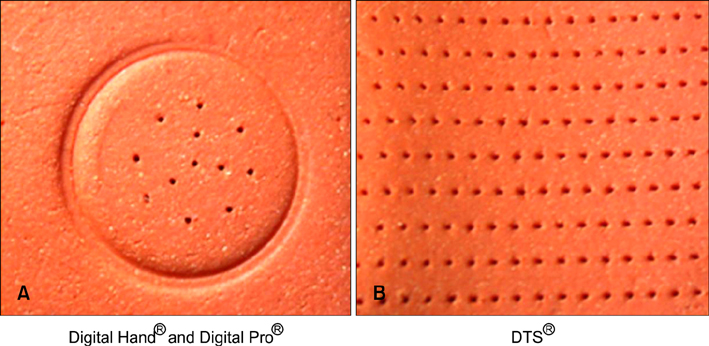
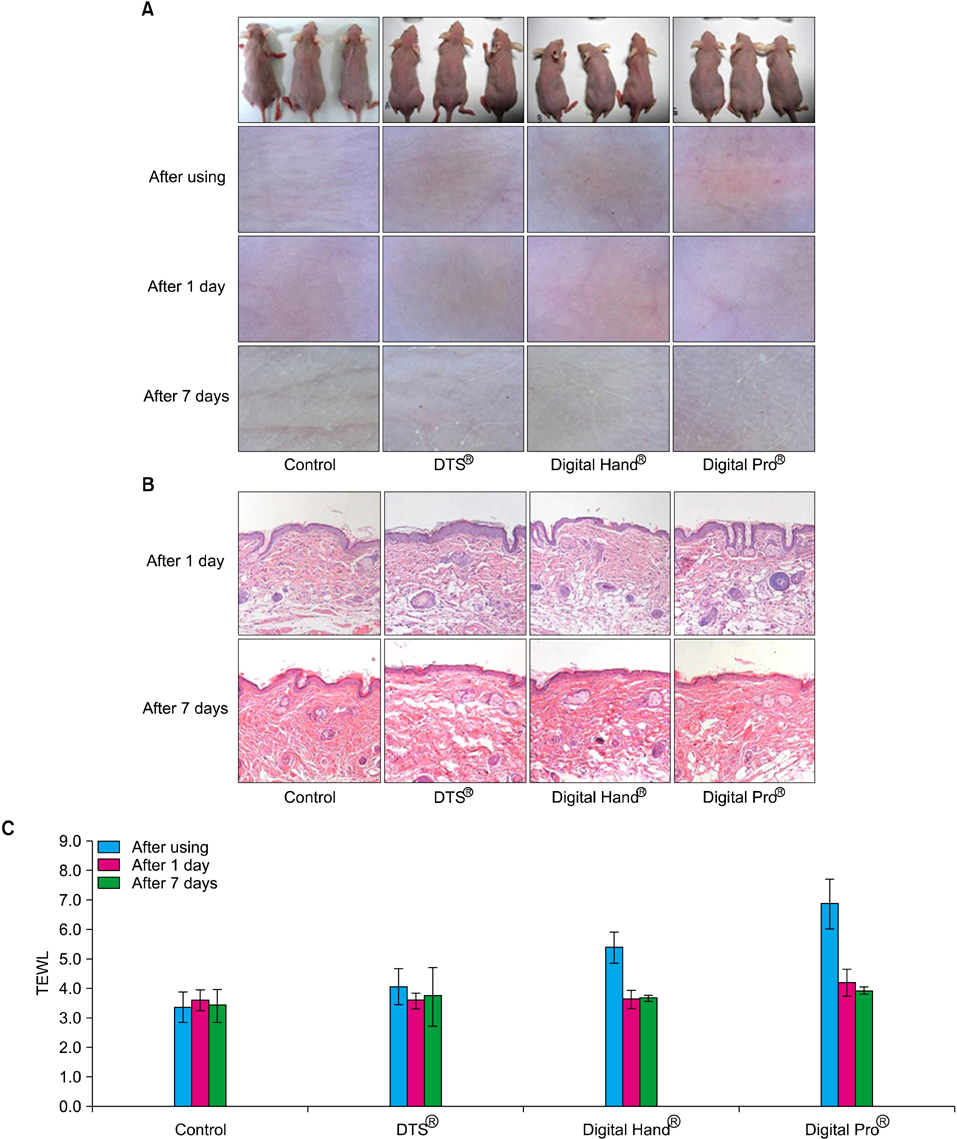
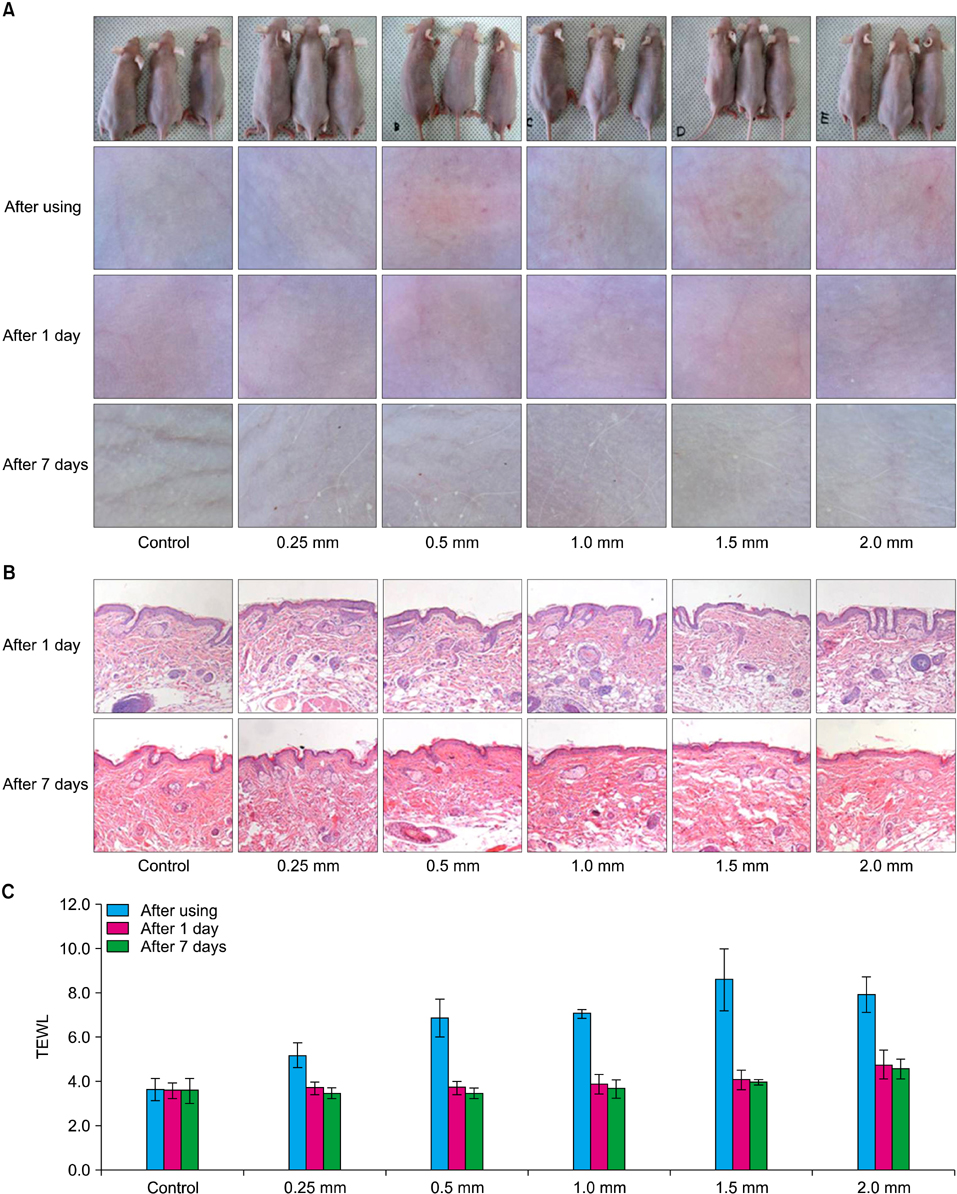
We used two newly developed digital microneedle devices to perforate the skin of skin-hairless-1 mice. We conducted a comparative study of the two digital microneedle devices and DTS(R) (Disk type-microneedle Therapy System; DTS lab., Seoul, Korea). To evaluate skin stability, we performed visual and dermatoscopic inspections, measurements of transepidermal water loss, and biopsies.
RESULTS
The two novel digital microneedle devices did not induce significant abnormalities of the skin on visual or dermatoscopic inspection, regardless of needle size (0.25~2.0 mm). No significant histopathological changes, such as inflammatory cell infiltration, desquamation of the stratum corneum, or disruption of the basal layer, were observed. The digital microneedle devices and microneedle therapy system produced similar results on measures of skin stability.
CONCLUSION
These two novel digital microneedle devices are safe transdermal drug delivery systems.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Repeated Microneedle Stimulation Induces Enhanced Hair Growth in a Murine Model
Yoon Seob Kim, Kwan Ho Jeong, Jung Eun Kim, Young Jun Woo, Beom Joon Kim, Hoon Kang
Ann Dermatol. 2016;28(5):586-592. doi: 10.5021/ad.2016.28.5.586.
Reference
-
1. Purdon CH, Azzi CG, Zhang J, Smith EW, Maibach HI. Penetration enhancement of transdermal delivery--current permutations and limitations. Crit Rev Ther Drug Carrier Syst. 2004. 21:97–132.
Article2. Touitou E. Drug delivery across the skin. Expert Opin Biol Ther. 2002. 2:723–733.
Article3. Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995. 269:850–853.
Article4. Badkar AV, Smith AM, Eppstein JA, Banga AK. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharm Res. 2007. 24:1389–1395.
Article5. Schuetz YB, Naik A, Guy RH, Kalia YN. Emerging strategies for the transdermal delivery of peptide and protein drugs. Expert Opin Drug Deliv. 2005. 2:533–548.
Article6. Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers-dermal and transdermal drug-delivery. J Control Release. 1994. 30:1–15.
Article7. Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004. 3:115–124.
Article8. Chen Y, Shen Y, Guo X, Zhang C, Yang W, Ma M, et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 2006. 24:455–460.
Article9. Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998. 87:922–925.
Article10. Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004. 56:581–587.
Article11. Kim K, Lee JB. High aspect ratio tapered hollow metallic microneedle arrays with microfluidic interconnector. Microsyst Technol. 2006. 13:231–235.
Article12. McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003. 100:13755–13760.
Article13. Oh JH, Park HH, Do KY, Han M, Hyun DH, Kim CG, et al. Influence of the delivery systems using a microneedle array on the permeation of a hydrophilic molecule, calcein. Eur J Pharm Biopharm. 2008. 69:1040–1045.
Article14. Coulman SA, Anstey A, Gateley C, Morrissey A, McLoughlin P, Allender C, et al. Microneedle mediated delivery of nanoparticles into human skin. Int J Pharm. 2009. 366:190–200.
Article15. Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009. 333:369–393.
Article16. Lin W, Cormier M, Samiee A, Griffin A, Johnson B, Teng CL, et al. Transdermal delivery of antisense oligonucleotides with microprojection patch (Macroflux) technology. Pharm Res. 2001. 18:1789–1793.17. Cormier M, Daddona PE. Rathbone MJ, Hadgraft J, Roberts MS, editors. Macroflux technology for transdermal delivery of therapeutic proteins and vaccines. Modifiedrelease drug delivery technology. 2003. New York: Marcel Dekker;589–598.
Article18. Vandervoort J, Ludwig A. Microneedles for transdermal drug delivery: a minireview. Front Biosci. 2008. 13:1711–1715.
Article19. Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002. 19:63–70.20. Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004. 97:503–511.
Article21. Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006. 24:1653–1664.
Article22. Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004. 21:947–952.
Article23. Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006. 126:1080–1087.
Article24. Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007. 14:375–381.
Article25. Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008. 24:585–594.
Article26. Roskos KV, Guy RH. Assessment of skin barrier function using transepidermal water loss: effect of age. Pharm Res. 1989. 6:949–953.27. Netzlaff F, Kostka KH, Lehr CM, Schaefer UF. TEWL measurements as a routine method for evaluating the integrity of epidermis sheets in static Franz type diffusion cells in vitro. Limitations shown by transport data testing. Eur J Pharm Biopharm. 2006. 63:44–50.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dermal Proliferative Effect and Safety of Automicroneedle Therapy System (AMTS)
- The Stability of the Disk-type Microneedle in Skh-hairless-1 (Albino) Mice
- Effects of Topical Application of Halofuginone on Wound Healing
- Clinical Significance of Nocturnal Penile Tumescence Monitoring with Stamps
- Effects of Light Pollution from Mobile Digital Devices on Sleep and Circadian Rhythms