Korean J Hematol.
2010 Mar;45(1):8-13. 10.5045/kjh.2010.45.1.8.
Recent advances in the management of venous thromboembolism
- Affiliations
-
- 1Research Center on Thromboembolic Diseases and Antithrombotic Therapies, Department of Clinical Medicine, University of Insubria, Varese, Italy. agewal@yahoo.com
- KMID: 2252077
- DOI: http://doi.org/10.5045/kjh.2010.45.1.8
Abstract
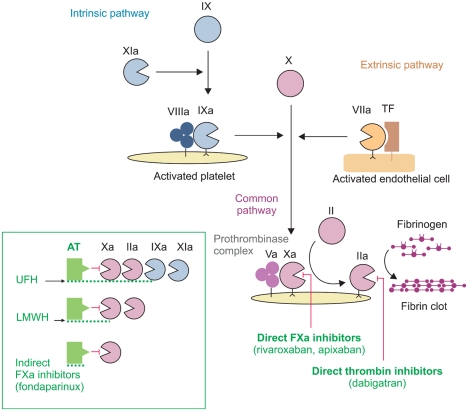
- Venous thromboembolism (VTE) is a spectrum of diseases that includes deep vein thrombosis (DVT) and pulmonary embolism (PE). Anticoagulant treatment is the mainstay of therapy for VTE. Unfractionated heparin (UFH) or low molecular weight heparin (LMWH) followed by vitamin K antagonists have been the treatment of choice for most patients with VTE, with the aim to prevent thrombus extension or embolization and recurrent VTE. Fondaparinux, a selective, indirect, parenteral factor Xa inhibitor, is now also approved for the initial treatment of VTE and represents an important alternative to UFH or LMWH. Secondary prevention of VTE with vitamin K antagonists is usually prescribed for a minimum of three months, with the duration of treatment based on the presence or absence of major identifiable risk factors for the index event. Patients with permanent risk factors or patients with recurrent DVT or PE require life long secondary prevention. Over the last years, new oral anticoagulant agents have been developed and are now undergoing extensive clinical evaluation in several settings, including the treatment of VTE. New oral anticoagulants include selective, direct thrombin inhibitors, such as dabigatran etexilate, and selective, direct factor Xa inhibitos, such as rivaroxaban, apixaban or edoxaban. All these drugs are admistered at fixed daily doses and do not require laboratory monitoring. The positive results of the first completed clinical trials suggest that a new era in the management of VTE is about to begin.
MeSH Terms
-
Anticoagulants
Antithrombins
Benzimidazoles
Factor Xa
Heparin
Heparin, Low-Molecular-Weight
Humans
Morpholines
Polysaccharides
Pulmonary Embolism
Pyrazoles
Pyridines
Pyridones
Risk Factors
Secondary Prevention
Thiazoles
Thiophenes
Thrombosis
Venous Thromboembolism
Venous Thrombosis
Vitamin K
Dabigatran
Rivaroxaban
Anticoagulants
Antithrombins
Benzimidazoles
Factor Xa
Heparin
Heparin, Low-Molecular-Weight
Morpholines
Polysaccharides
Pyrazoles
Pyridines
Pyridones
Thiazoles
Thiophenes
Vitamin K
Figure
Reference
-
1. Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism: a controlled trial. Lancet. 1960; 1:1309–1312. PMID: 13797091.2. Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: american college of chest physicians evidence-based clinical practice guidelines (8th Edition). Chest. 2008; 133(6 Suppl):454S–545S. PMID: 18574272.3. Büller HR, Davidson BL, Decousus H, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004; 140:867–873. PMID: 15172900.4. Büller HR, Davidson BL, Decousus H, et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003; 349:1695–1702. PMID: 14585937.
Article5. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002; 347:1143–1150. PMID: 12374874.
Article6. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the european society of cardiology (ESC). Eur Heart J. 2008; 29:2276–2315. PMID: 18757870.7. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003; 349:146–153. PMID: 12853587.
Article8. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002; 162:1729–1735. PMID: 12153376.9. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006; 119:1062–1072. PMID: 17145251.
Article10. White RH. The epidemiology of venous thromboembolism. Circulation. 2003; 107:I4–I8. PMID: 12814979.
Article11. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006; 355:1780–1789. PMID: 17065639.
Article12. Cosmi B, Legnani C, Tosetto A, et al. Usefulness of repeated D-dimer testing after stopping anticoagulation for a first episode of unprovoked venous thromboembolism: the PROLONG II prospective study. Blood. 2010; 115:481–488. PMID: 19965693.
Article13. Eichinger S, Minar E, Bialonczyk C, et al. D-dimer levels and risk of recurrent venous thromboembolism. JAMA. 2003; 290:1071–1074. PMID: 12941680.
Article14. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002; 137:955–960. PMID: 12484710.
Article15. Siragusa S, Malato A, Anastasio R, et al. Residual vein thrombosis to establish duration of anticoagulation after a first episode of deep vein thrombosis: the Duration of Anticoagulation based on Compression UltraSonography (DACUS) study. Blood. 2008; 112:511–515. PMID: 18497320.
Article16. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009; 150:577–585. PMID: 19414836.
Article17. Turpie AGG. Oral, direct Factor Xa inhibitors in development for the prevention and treatment of thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2007; 27:1238–1247. PMID: 17379841.
Article18. Weitz JI, Hirsh J, Samama MM. New antithrombotic drugs: American college of chest physicians evidence-based clinical practice guidelines (8th Edition). Chest. 2008; 133:234S–256S. PMID: 18574267.19. European public assessment report: Pradaxa. 2008. Accessed January 10, 2010. London: European Medicines Agency (EMEA);http://www.emea.europa.eu.20. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009; 361:2342–2352. PMID: 19966341.
Article21. Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety pharmacodynamics and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005; 78:412–421. PMID: 16198660.
Article22. Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet. 2009; 48:1–22. PMID: 19071881.23. Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety pharmacodynamics and pharmacokinetics of BAY59-7939-an oral, direct Factor Xa inhibitor-after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005; 61:873–880. PMID: 16328318.24. European public assessment report: Xarelto. 2008. Accessed January 10, 2010. London: European Medicines Agency (EMEA);http://www.emea.europa.eu.25. Agnelli G, Gallus A, Goldhaber SZ, et al. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY59-7939): the ODIXa DVT (oral direct factor Xa inhibitor BAY59-7939 in patients with acute symptomatic deep-vein thrombosis) study. Circulation. 2007; 116:180–187. PMID: 17576867.26. Buller HR, Lensing AW, Prins MH, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging Study. Blood. 2008; 112:2242–2247. PMID: 18621928.
Article27. Buller HR. Once daily oral rivaroxaban versus placebo in the long term prevention of recurrent symptomatic venous thromboembolism. The Einstein-Extension study. Blood. 2009; 114:Abstract 2.[U01].28. Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost. 2008; 6:820–829. PMID: 18315548.29. Wang L, Zhang D, Raghavan N, et al. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Dispos. 2010; 38:448–458. PMID: 19940026.
Article30. Buller H, Deitchman D, Prins M, et al. Efficacy and safety of the oral direct facto Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost. 2008; 6:1313–1318. PMID: 18541000.