Korean J Hematol.
2010 Dec;45(4):269-274. 10.5045/kjh.2010.45.4.269.
The use of the complement inhibitor eculizumab (Soliris(R)) for treating Korean patients with paroxysmal nocturnal hemoglobinuria
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, The Catholic University of Korea, Seoul St. Mary's Hospital, Seoul, Korea. jwlee@catholic.ac.kr
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Hematology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Hematology-Oncology, Pusan National University Medical School, Pusan National University Hospital, Busan, Korea.
- KMID: 2252040
- DOI: http://doi.org/10.5045/kjh.2010.45.4.269
Abstract
- BACKGROUND
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired clonal disorder characterized by chronic complement-mediated hemolysis. Eculizumab, a humanized monoclonal antibody against the terminal complement protein C5, potently reduces chronic intravascular hemolysis. We tested the clinical efficacy and safety of a 24-week treatment with eculizumab in 6 Korean patients with PNH.
METHODS
We enrolled 6 patients with PNH who had clinically significant hemolysis. Eculizumab was administered intravenously at 600 mg/week for the first 4 weeks followed by 900 mg at week 5 and 2nd weekly thereafter.
RESULTS
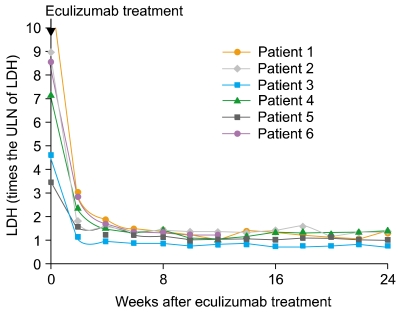
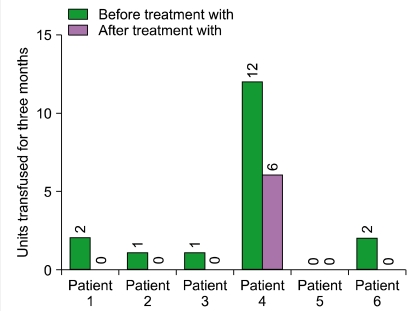
Three men and 3 women with a median age of 39.5 years (24-61 years) were enrolled. The median duration of PNH was 11 years (6-25 years). Hemolysis occurred in all patients [median lactate dehydrogenase (LDH) level, 7.95 times the upper limit of the reference range of LDH]. All patients treated with eculizumab had a rapid and sustained reduction in the degree of hemolysis. RBC transfusion requirements for 3 months were decreased from 0-12 units (median requirement, 1.5 units) to 0-6 units (median requirement, 0 units). Improvement in fatigue was noted in 4 patients. Further, 5 patients who had been receiving corticosteroids either reduced the dose or discontinued therapy. No significant adverse events related to eculizumab therapy were observed.
CONCLUSION
These results show that eculizumab reduces the degree of intravascular hemolysis, reduces or eliminates the requirement of RBC transfusion, and improves anemia and fatigue in patients with PNH. Eculizumab is an effective and safe option for treating Korean patients with PNH.
MeSH Terms
Figure
Reference
-
1. Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995; 333:1253–1258. PMID: 7566002.
Article2. Nishimura J, Kanakura Y, Ware RE, et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore). 2004; 83:193–207. PMID: 15118546.
Article3. de Latour RP, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008; 112:3099–3106. PMID: 18535202.
Article4. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005; 106:3699–3709. PMID: 16051736.
Article5. Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009; 113:6522–6527. PMID: 19372253.
Article6. Lee JW, Jang JH, Lee JH, et al. High prevalence and mortality associated with thromboembolism in Asian patients with paroxysmal nocturnal hemoglobinuria (PNH). Haematologica. 2010; 95(suppl 2):205. (abst 505).7. Lee JW, Jang JH, Lee JH, et al. Clinical symptoms of hemolysis are predictive of disease burden and mortality in Asian patients with paroxysmal nocturnal hemoglobinuria (PNH). Haematologica. 2010; 95(suppl 2):206. (abst 506). PMID: 19773262.8. Parker C. Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet. 2009; 373:759–767. PMID: 19144399.
Article9. Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004; 350:552–559. PMID: 14762182.
Article10. Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006; 355:1233–1243. PMID: 16990386.
Article11. Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008; 111:1840–1847. PMID: 18055865.
Article12. Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System. 1997. 4th ed. Evanston, IL: Center on Outcomes Research and Education (CORE), Evanston Northwestern Healthcare and Northwestern University;p. 13–19.13. Saso R, Marsh J, Cevreska L, et al. Bone marrow transplants for paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1999; 104:392–396. PMID: 10050724.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anesthetic and Perioperative Management of Patient with Paroxysmal Nocturnal Hemoglobinuria
- A Successful Planned Pregnancy and Delivery with Eculizumab Maintenance in a Woman with Paroxysmal Nocturnal Hemoglobinuria
- Paroxysmal Nocturnal Hemoglobinuria Presenting as Recurrent Jejunitis
- Delivery in a Patient with Paroxysmal Nocturnal Hemoglobinuria Successfully Managed with Low Molecular Weight Heparin Therapy
- A Case of Paroxysmal Nocturnal Hemoglobinuria