Korean J Hematol.
2010 Dec;45(4):224-235. 10.5045/kjh.2010.45.4.224.
The immunobiology of cord blood transplantation
- Affiliations
-
- 1Department of Pediatrics, Pediatric Blood and Marrow Transplant Program and Department of Immunology, Duke University, Durham, USA. szabo001@mc.duke.edu
- KMID: 2252032
- DOI: http://doi.org/10.5045/kjh.2010.45.4.224
Abstract
- Despite significant recent advances in the applicability and outcome following unrelated cord blood transplantation (UCBT), infections remain a major cause of mortality associated with poor immune recovery in the first 6 months after UCBT. Enhanced immune reconstitution not only could improve survival by reduced transplant related mortality, but may also favorably impact on relapse incidence by improved graft-versus-leukemia effects. This review will summarize our current understanding of the biology of immune recovery post-UCBT with an emphasis on adaptive T cell dependent immunity. New efforts to boost immunity will be also highlighted including our own laboratory, where ex vivo T cell expansion is pursued towards adoptive immunotherapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effect of Human Parathyroid Hormone on Hematopoietic Progenitor Cells in NOD/SCID Mice Co-Transplanted with Human Cord Blood Mononuclear Cells and Mesenchymal Stem Cells
Yeon-Jung Lim, Kyoujung Hwang, Miyeon Kim, Youl-Hee Cho, Jong-Hwa Lee, Young-Ho Lee, Jong-Jin Seo
Yonsei Med J. 2013;54(1):238-245. doi: 10.3349/ymj.2013.54.1.238.
Reference
-
1. Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968; 2:1366–1369. PMID: 4177932.2. Bach FH, Albertini RJ, Joo P, Anderson JL, Bortin MM. Bone-marrow transplantation in a patient with the Wiskott-Aldrich syndrome. Lancet. 1968; 2:1364–1366. PMID: 4177931.
Article3. Ende M, Ende N. Hematopoietic transplantation by means of fetal (cord) blood. A new method. Va Med Mon (1918). 1972; 99:276–280. PMID: 4502979.4. Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989; 86:3828–3832. PMID: 2566997.
Article5. Gluckman E, Rocha V. History of the clinical use of umbilical cord blood hematopoietic cells. Cytotherapy. 2005; 7:219–227. PMID: 16081348.
Article6. Broxmeyer HE. Biology of cord blood cells and future prospects for enhanced clinical benefit. Cytotherapy. 2005; 7:209–218. PMID: 16081347.
Article7. Leung W, Ramírez M, Civin CI. Quantity and quality of engrafting cells in cord blood and autologous mobilized peripheral blood. Biol Blood Marrow Transplant. 1999; 5:69–76. PMID: 10371358.
Article8. Frassoni F, Podesta M, Maccario R, et al. Cord blood transplantation provides better reconstitution of hematopoietic reservoir compared with bone marrow transplantation. Blood. 2003; 102:1138–1141. PMID: 12689932.
Article9. Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989; 321:1174–1178. PMID: 2571931.
Article10. Kurtzberg J, Graham M, Casey J, Olson J, Stevens CE, Rubinstein P. The use of umbilical cord blood in mismatched related and unrelated hemopoietic stem cell transplantation. Blood Cells. 1994; 20:275–283. PMID: 7749107.11. Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009; 94:451–454. PMID: 19336748.
Article12. Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001; 97:2957–2961. PMID: 11342417.
Article13. Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001; 97:2962–2971. PMID: 11342418.
Article14. Rocha V, Gluckman E. Eurocord and European Blood and Marrow Transplant Group. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006; 12(1 Suppl 1):34–41. PMID: 16399582.
Article15. Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998; 339:1565–1577. PMID: 9828244.
Article16. Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004; 351:2276–2285. PMID: 15564544.
Article17. Kurtzberg J, Carter SL, Baxter-Lowe LA, et al. Results of the cord blood transplantation study (COBLT): Clinical outcomes of 193 unrelated donor umbilical cord blood transplantation in pediatric patients with malignant conditions. Biol Blood Marrow Transplant. 2005; 11(Suppl 1):2. PMID: 15682165.
Article18. Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004; 351:2265–2275. PMID: 15564543.
Article19. Parody R, Martino R, Rovira M, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006; 12:734–748. PMID: 16785063.
Article20. Barker JN, Hough RE, van Burik JA, et al. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005; 11:362–370. PMID: 15846290.
Article21. Cohen G, Carter SL, Weinberg KI, et al. Antigen-specific T-lymphocyte function after cord blood transplantation. Biol Blood Marrow Transplant. 2006; 12:1335–1342. PMID: 17162216.
Article22. Parkman R, Weinberg KI. Immunological reconstitution following bone marrow transplantation. Immunol Rev. 1997; 157:73–78. PMID: 9255623.
Article23. Storek J, Geddes M, Khan F, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008; 30:425–437. PMID: 18949477.
Article24. Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996; 156:4609–4616. PMID: 8648103.25. Fry TJ, Mackall CL. Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant. 2005; 35(Suppl 1):S53–S57. PMID: 15812532.
Article26. Crooks GM, Weinberg K, Mackall C. Immune reconstitution: from stem cells to lymphocytes. Biol Blood Marrow Transplant. 2006; 12(1 Suppl 1):42–46. PMID: 16399583.
Article27. Talvensaari K, Clave E, Douay C, et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. 2002; 99:1458–1464. PMID: 11830500.
Article28. Han P, Hodge G, Story C, Xu X. Phenotypic analysis of functional T-lymphocyte subtypes and natural killer cells in human cord blood: relevance to umbilical cord blood transplantation. Br J Haematol. 1995; 89:733–740. PMID: 7772509.
Article29. D'Arena G, Musto P, Cascavilla N, et al. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998; 83:197–203. PMID: 9573672.30. Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naïve phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol. 2003; 31:708–714. PMID: 12901976.
Article31. Broxmeyer HE, editor. Cord blood: biology, immunology, banking and clinical transplantation. 2004. Bethesda, Md: American Association of Blood Banks.32. Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996; 184:539–548. PMID: 8760807.
Article33. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998; 281:1191–1193. PMID: 9712583.
Article34. Szekeres-Bartho J, Faust Z, Varga P, Szereday L, Kelemen K. The immunological pregnancy protective effect of progesterone is manifested via controlling cytokine production. Am J Reprod Immunol. 1996; 35:348–351. PMID: 8739452.
Article35. Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. 2005; 141:10–18. PMID: 15958064.
Article36. Guller S, LaChapelle L. The role of placental Fas ligand in maintaining immune privilege at maternal-fetal interfaces. Semin Reprod Endocrinol. 1999; 17:39–44. PMID: 10406074.37. Ribeiro-do-Couto LM, Boeije LC, Kroon JS, et al. High IL-13 production by human neonatal T cells: neonate immune system regulator? Eur J Immunol. 2001; 31:3394–3402. PMID: 11745358.
Article38. White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002; 168:2820–2827. PMID: 11884451.39. Goriely S, Van Lint C, Dadkhah R, et al. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004; 199:1011–1016. PMID: 15051764.
Article40. Goriely S, Vincart B, Stordeur P, et al. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001; 166:2141–2146. PMID: 11160266.
Article41. Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002; 128:118–123. PMID: 11982599.
Article42. Tu W, Chen S, Sharp M, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004; 172:3260–3267. PMID: 14978134.43. Suen Y, Lee SM, Qian J, van de Ven C, Cairo MS. Dysregulation of lymphokine production in the neonate and its impact on neonatal cell mediated immunity. Vaccine. 1998; 16:1369–1377. PMID: 9711774.
Article44. Bradley MB, Cairo MS. Cord blood immunology and stem cell transplantation. Hum Immunol. 2005; 66:431–446. PMID: 15935881.
Article45. Berthou C, Legros-Maïda S, Soulié A, et al. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. 1995; 85:1540–1546. PMID: 7534135.
Article46. Park KD, Marti L, Kurtzberg J, Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006; 108:1770–1773. PMID: 16675712.47. Takahata Y, Nomura A, Takada H, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004; 32:622–629. PMID: 15246158.
Article48. Godfrey WR, Spoden DJ, Ge YG, et al. Cord blood CD4(+) CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005; 105:750–758. PMID: 15374887.49. Schönland SO, Zimmer JK, Lopez-Benitez CM, et al. Homeostatic control of T-cell generation in neonates. Blood. 2003; 102:1428–1434. PMID: 12714521.50. Gardiner CM, Meara AO, Reen DJ. Differential cytotoxicity of cord blood and bone marrow-derived natural killer cells. Blood. 1998; 91:207–213. PMID: 9414286.
Article51. Harris DT, Schumacher MJ, Locascio J, et al. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992; 89:10006–10010. PMID: 1438190.
Article52. Klein AK, Patel DD, Gooding ME, et al. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2001; 7:454–466. PMID: 11569891.
Article53. Moretta A, Maccario R, Fagioli F, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol. 2001; 29:371–379. PMID: 11274766.
Article54. Brahmi Z, Hommel-Berrey G, Smith F, Thomson B. NK cells recover early and mediate cytotoxicity via perforin/granzyme and Fas/FasL pathways in umbilical cord blood recipients. Hum Immunol. 2001; 62:782–790. PMID: 11476901.
Article55. Rénard C, Barlogis V, Mialou V, et al. Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. Br J Haematol. 2010; [Epub ahead of print].
Article56. Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000; 96:2703–2711. PMID: 11023501.
Article57. Koh LP, Chao NJ. Umbilical cord blood transplantation in adults using myeloablative and nonmyeloablative preparative regimens. Biol Blood Marrow Transplant. 2004; 10:1–22. PMID: 14752775.
Article58. Parkman R, Cohen G, Carter SL, et al. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006; 12:919–927. PMID: 16920557.
Article59. Burke MJ, Vogel RI, Janardan SK, et al. Early Lymphocyte Recovery and Outcomes after Umbilical Cord Blood Transplantation (UCBT) for Hematologic Malignancies. Biol Blood Marrow Transplant. 2010; [Epub ahead of print].
Article60. Szabolcs P, Niedzwiecki D, Chao N, Kurtzberg J. Multivariate analysis of patient and graft specific factors among 330 recipients of unrelated cord blood transplant (UCBT) to predict risk of death from opportunistic infections in the first 6 months after UCBT. Blood. 2006; 108(11):(abst 2860).
Article61. Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007; 9:111–122. PMID: 17453963.
Article62. Szabolcs P, Park KD, Marti L, et al. The impact of immune reconstitution in the early post grafting period on the development of opportunistic infections after unrelated cord blood transplantation: a multivariate analysis of host, graft, and day +50 immune profile. Biol Blood Marrow Transplant. 2004; 10(Suppl 1):24(abst 48).
Article63. Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Absolute values of dendritic cell subsets in bone marrow, cord blood, and peripheral blood enumerated by a novel method. Stem Cells. 2003; 21:296–303. PMID: 12743324.
Article64. Szabolcs P, Park KD, Marti L, et al. Superior depletion of alloreactive T cells from peripheral blood stem cell and umbilical cord blood grafts by the combined use of trimetrexate and interleukin-2 immunotoxin. Biol Blood Marrow Transplant. 2004; 10:772–783. PMID: 15505608.
Article65. Szabolcs P, Lee YA, Reese M, Chao N, Kurtzberg J. Rapid In Vivo Acquisition of Effector Tc1 Phenotype Prior to Myeloid Engraftment Predicts Opportunistic Infections in Unrelated Cord Blood Transplant Recipients. Biol Blood Marrow Transplant. 2006; 12:83–84.
Article66. Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997; 186:1407–1418. PMID: 9348298.
Article67. Mazur MA, Davis CC, Szabolcs P. Ex vivo expansion and Th1/Tc1 maturation of umbilical cord blood T cells by CD3/CD28 costimulation. Biol Blood Marrow Transplant. 2008; 14:1190–1196. PMID: 18804050.
Article68. Parmar S, Robinson SN, Komanduri K, et al. Ex vivo expanded umbilical cord blood T cells maintain naive phenotype and TCR diversity. Cytotherapy. 2006; 8:149–157. PMID: 16698688.
Article69. June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990; 11:211–216. PMID: 2162180.
Article70. Levine BL, Bernstein WB, Aronson NE, et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002; 8:47–53. PMID: 11786906.
Article71. Laport GG, Levine BL, Stadtmauer EA, et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003; 102:2004–2013. PMID: 12763934.
Article72. Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006; 107:1325–1331. PMID: 16269610.
Article73. Hagihara M, Chargui J, Gansuvd B, et al. Umbilical cord blood T lymphocytes are induced to apoptosis after being allo-primed in vitro. Bone Marrow Transplant. 1999; 24:1229–1233. PMID: 10642813.
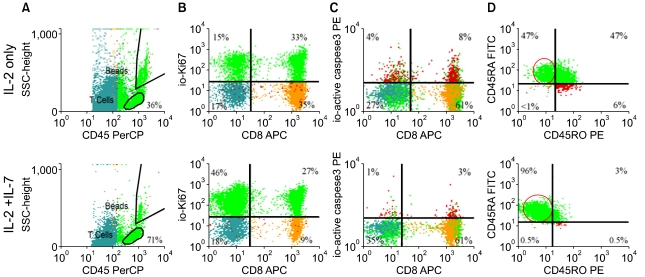
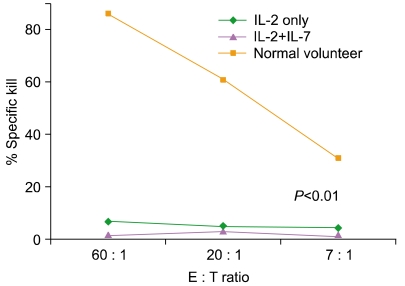
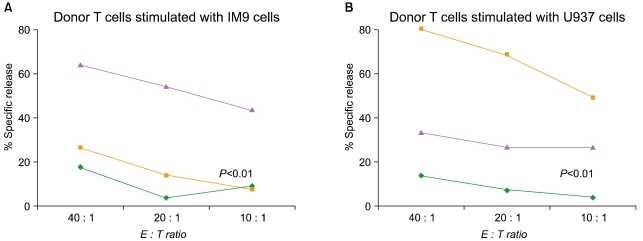
Article74. Davis CC, Marti LC, Sempowski GD, Jeyaraj DA, Szabolcs P. Interleukin-7 permits Th1/Tc1 maturation and promotes ex vivo expansion of cord blood T cells: a critical step toward adoptive immunotherapy after cord blood transplantation. Cancer Res. 2010; 70:5249–5258. PMID: 20530666.
Article75. Snyder KM, Mackall CL, Fry TJ. IL-7 in allogeneic transplant: clinical promise and potential pitfalls. Leuk Lymphoma. 2006; 47:1222–1228. PMID: 16923550.
Article76. Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006; 211:154–163. PMID: 16824125.
Article77. Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999; 23:783–788. PMID: 10231140.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Umbilical Cord Blood Transplantation
- Umbilical Cord Blood Stem Cell Transplantation
- Cord Blood-Current Status and Perspective
- Current Status for Cord Blood Transplantation and Public Cord Blood Bank in Korea
- StemCell Transplantation in Umbilical Cord Blood(II) Characteristics of T-Lymphocyte Subpopulation in Umbilical Cord Blood