J Clin Neurol.
2015 Apr;11(2):188-191. 10.3988/jcn.2015.11.2.188.
The Overlap between Fibromyalgia Syndrome and Myotonia Congenita
- Affiliations
-
- 1Department of Neurology, Chonnam National University Medical School, Gwangju, Korea. mkkim@jnu.ac.kr
- 2Department of Neurology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Department of Biomedical Sciences, Chonnam National University Medical School, Gwangju, Korea.
- 4Department of Rheumatology, Chonnam National University Medical School, Gwangju, Korea.
- 5Department of Pediatrics, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2242665
- DOI: http://doi.org/10.3988/jcn.2015.11.2.188
Abstract
- BACKGROUND
Fibromyalgia syndrome (FMS) is a complex disorder characterized by chronic widespread pain (CWP), multiple areas of tenderness, sleep disturbance, fatigue, and mood or cognitive dysfunction. Myotonia congenita (MC) is an inherited myopathic disorder that is caused by mutations in the gene encoding the skeletal muscle chloride channel, which can infrequently manifest as generalized muscle cramps or myalgia.
CASE REPORT
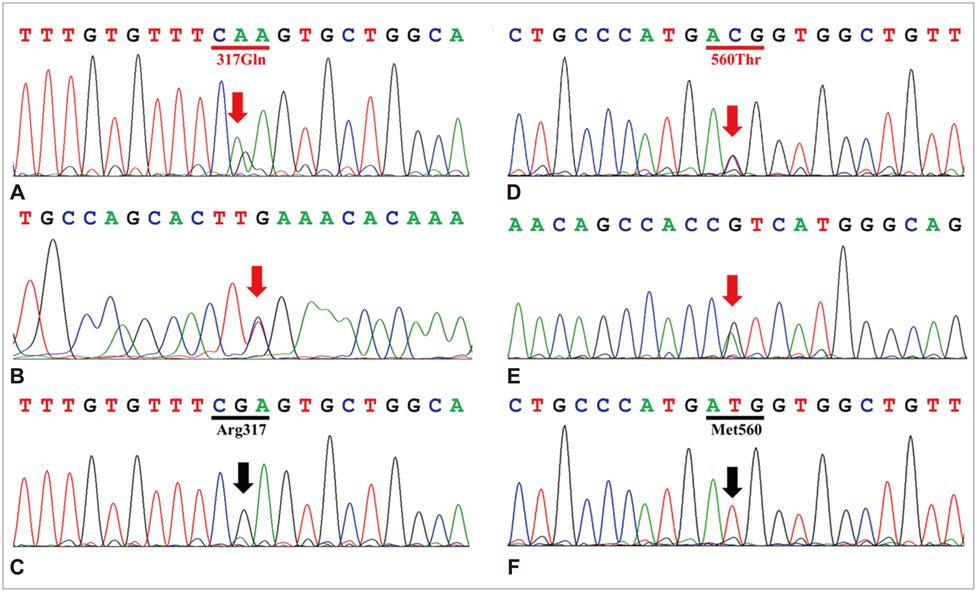
The first case was a 33-year-old woman who complained of CWP and chronic headache occurring during pregnancy, and the second case was a 37-year-old man with CWP and depression who suffered from cold-induced muscle cramps. These two patients were initially diagnosed with FMS by rheumatologists, based on CWP of longer than 3 months duration and mechanical tenderness in specific body regions. However, these two FMS patients were subsequently also diagnosed with MC.
CONCLUSIONS
These two cases are the first report of an overlap of CWP between FMS and MC.
Keyword
MeSH Terms
Figure
Reference
-
1. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990; 33:160–172.
Article2. Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995; 38:19–28.
Article3. Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, et al. Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum. 2010; 39:448–453.
Article4. Amato AA, Russell JA. Nondystrophic myotonias and periodic paralysis. In : Amato AA, Russell JA, editors. Neuromuscular Disorders. New York: McGraw;2008. p. 655–680.5. Lossin C, George AL Jr. Myotonia congenita. Adv Genet. 2008; 63:25–55.6. Moon IS, Kim HS, Shin JH, Park YE, Park KH, Shin YB, et al. Novel CLCN1 mutations and clinical features of Korean patients with myotonia congenita. J Korean Med Sci. 2009; 24:1038–1044.
Article7. Becker RM, da Silva VK, Machado Fda S, dos Santos AF, Meireles DC, Mergener M, et al. Association between environmental quality, stress and APOE gene variation in fibromyalgia susceptibility determination. Rev Bras Reumatol. 2010; 50:617–624.8. Day JW, Ricker K, Jacobsen JF, Rasmussen LJ, Dick KA, Kress W, et al. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology. 2003; 60:657–664.9. Udd B, Meola G, Krahe R, Thornton C, Ranum LP, Bassez G, et al. 140th ENMC International Workshop: Myotonic Dystrophy DM2/PROMM and other myotonic dystrophies with guidelines on management. Neuromuscul Disord. 2006; 16:403–413.
Article10. Götze FR, Thid S, Kyllerman M. Fibromyalgia in hyperkalemic periodic paralysis. Scand J Rheumatol. 1998; 27:383–384.11. Auvinen S, Suominen T, Hannonen P, Bachinski LL, Krahe R, Udd B. Myotonic dystrophy type 2 found in two of sixty-three persons diagnosed as having fibromyalgia. Arthritis Rheum. 2008; 58:3627–3631.
Article12. Saa'd S, Many A, Jacob G, Ablin JN. High prevalence of fibromyalgia symptoms among healthy full-term pregnant women. Rheumatol Int. 2013; 33:1555–1560.13. Schwartz IL, Dingfelder JR, O'Tuama L, Swift M. Recessive congenital myotonia and pregnancy. Int J Gynaecol Obstet. 1979; 17:194–196.
Article14. Basu A, Nishanth P, Ifaturoti O. Pregnancy in women with myotonia congenita. Int J Gynaecol Obstet. 2009; 106:62–63.
Article15. Berglund B, Harju EL, Kosek E, Lindblom U. Quantitative and qualitative perceptual analysis of cold dysesthesia and hyperalgesia in fibromyalgia. Pain. 2002; 96:177–187.
Article16. Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, Mullins PG. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain. 2008; 140:420–428.
Article17. Lee SC, Kim HS, Park YE, Choi YC, Park KH, Kim DS. Clinical Diversity of SCN4A-Mutation-Associated Skeletal Muscle Sodium Channelopathy. J Clin Neurol. 2009; 5:186–191.
Article