J Clin Neurol.
2015 Apr;11(2):172-177. 10.3988/jcn.2015.11.2.172.
Preclinical Assessment of the Anticancer Drug Response of Plexiform Neurofibroma Tissue Using Primary Cultures
- Affiliations
-
- 1Department of Neurology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. w.jiang@uke.de
- 2Department of Oral and Maxillofacial Surgery, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
- KMID: 2242662
- DOI: http://doi.org/10.3988/jcn.2015.11.2.172
Abstract
- BACKGROUND AND PURPOSE
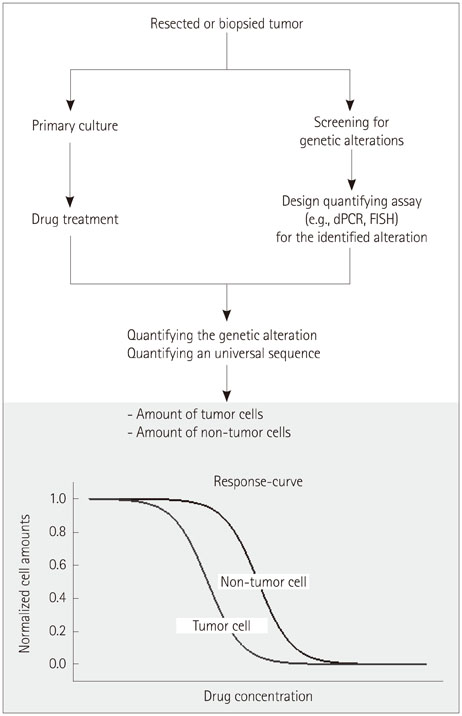
Individualized drug testing for tumors using a strategy analogous to antibiotic tests for infectious diseases would be highly desirable for personalized and individualized cancer care.
METHODS
Primary cultures containing tumor and nontumor stromal cells were utilized in a novel strategy to test drug responses with respect to both efficacy and specificity. The strategy tested in this pilot study was implemented using four primary cultures derived from plexiform neurofibromas. Responses to two cytotoxic drugs (nilotinib and imatinib) were measured by following dose-dependent changes in the proportions of tumor and nontumor cells, determined by staining them with cell-type-specific antibodies. The viability of the cultured cells and the cytotoxic effect of the drugs were also measured using proliferation and cytotoxicity assays.
RESULTS
The total number of cells decreased after the drug treatment, in accordance with the observed reduction in proliferation and increased cytotoxic effect upon incubation with the two anticancer drugs. The proportions of Schwann cells and fibroblasts changed dose-dependently, although the patterns of change varied between the tumor samples (from different sources) and between the two drugs. The highly variable in vitro drug responses probably reflect the large variations in the responses of tumors to therapies between individual patients in vivo.
CONCLUSIONS
These preliminary results suggest that the concept of assessing in vitro drug responses using primary cultures is feasible, but demands the extensive further development of an application for preclinical drug selection and drug discovery.
Keyword
MeSH Terms
Figure
Reference
-
1. Gonzalez de Castro D, Clarke PA, Al-Lazikani B, Workman P. Personalized cancer medicine: molecular diagnostics, predictive biomarkers, and drug resistance. Clin Pharmacol Ther. 2013; 93:252–259.
Article2. Meric-Bernstam F, Mills GB. Overcoming implementation challenges of personalized cancer therapy. Nat Rev Clin Oncol. 2012; 9:542–548.
Article3. Morabito F, Recchia AG, Mazzone C, Gentile M. Targeted therapy of multiple myeloma: the changing paradigm at the beginning of the new millennium. Curr Cancer Drug Targets. 2012; 12:743–756.
Article4. Eramo A, Haas TL, De Maria R. Lung cancer stem cells: tools and targets to fight lung cancer. Oncogene. 2010; 29:4625–4635.
Article5. Caponigro G, Sellers WR. Advances in the preclinical testing of cancer therapeutic hypotheses. Nat Rev Drug Discov. 2011; 10:179–187.
Article6. McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov. 2013; 12:217–228.
Article7. Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch Dermatol. 2005; 141:71–74.
Article8. Ferner RE, Gutmann DH. Neurofibromatosis type 1 (NF1): diagnosis and management. Handb Clin Neurol. 2013; 115:939–955.9. Mautner VF, Hartmann M, Kluwe L, Friedrich RE, Fünsterer C. MRI growth patterns of plexiform neurofibromas in patients with neurofibromatosis type 1. Neuroradiology. 2006; 48:160–165.
Article10. Nguyen R, Kluwe L, Fuensterer C, Kentsch M, Friedrich RE, Mautner VF. Plexiform neurofibromas in children with neurofibromatosis type 1: frequency and associated clinical deficits. J Pediatr. 2011; 159:652–655.e2.
Article11. Evans DG, Huson SM, Birch JM. Malignant peripheral nerve sheath tumours in inherited disease. Clin Sarcoma Res. 2012; 2:17.
Article12. Nguyen R, Ibrahim C, Friedrich RE, Westphal M, Schuhmann M, Mautner VF. Growth behavior of plexiform neurofibromas after surgery. Genet Med. 2013; 15:691–697.
Article13. Robertson KA, Nalepa G, Yang FC, Bowers DC, Ho CY, Hutchins GD, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 2012; 13:1218–1224.
Article14. Jiang W, Schnabel C, Spyra M, Mautner VF, Friedrich RE, Hagel C, et al. Efficacy and selectivity of nilotinib on NF1-associated tumors in vitro. J Neurooncol. 2014; 116:231–236.
Article15. Wei J, Freytag M, Schober Y, Nockher WA, Mautner VF, Friedrich RE, et al. Nilotinib is more potent than imatinib for treating plexiform neurofibroma in vitro and in vivo. PLoS One. 2014; 9:e107760.
Article16. Kluwe L, Friedrich R, Mautner VF. Loss of NF1 allele in Schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes Chromosomes Cancer. 1999; 24:283–285.
Article17. Serra E, Rosenbaum T, Winner U, Aledo R, Ars E, Estivill X, et al. Schwann cells harbor the somatic NF1 mutation in neurofibromas: evidence of two different Schwann cell subpopulations. Hum Mol Genet. 2000; 9:3055–3064.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Solitary Plexiform Neurofibroma on the Median Nerve: A Case Report
- A Case of Plexiform Neurofibroma with Multiple Skeletal Abnormalities and Giant Pigmentation
- A Case of Plexiform Neurofibroma Developed under the Overlying Speckled Lentiginous Nevus
- A Case of Isolated Plexiform Neurofibroma
- Neurofibroma Associated with Alopecia