J Breast Cancer.
2012 Jun;15(2):197-202. 10.4048/jbc.2012.15.2.197.
Impact of Triple-Negative Breast Cancer Phenotype on Prognosis in Patients with Stage I Breast Cancer
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. drjiny@amc.seoul.kr
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2242190
- DOI: http://doi.org/10.4048/jbc.2012.15.2.197
Abstract
- PURPOSE
Although most patients with stage I breast cancer have a good prognosis, their clinical outcomes may vary significantly. We assessed clinical outcomes and prognostic factors in stage I breast cancer patients with and without triple-negative breast cancer (TNBC) phenotype.
METHODS
Of 2,489 patients undergoing breast cancer surgery between January 1998 and December 2002, 554 (22.3%) had stage I breast cancer (tumor size < or =2 cm, and lymph node-negative). TNBC was defined as a primary tumor negative for estrogen and progesterone receptors (Allred scores <3/8) and for HER2/neu (0-1+ by immunohistochemistry).
RESULTS
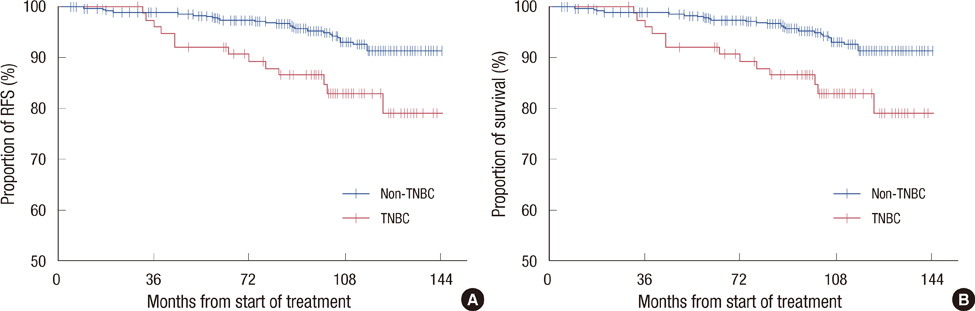
Of the 554 patients with stage I breast cancer, 78 (14.1%) had TNBC. A significant proportion of TNBC patients had histologic grade 3 tumors (47.4% vs. 34.5%, p=0.031) and tumors >1 cm (87.2% vs. 75.8%, p=0.028) and received adjuvant chemotherapy (79.5% vs. 44.7%, p<0.001). During a median follow-up time of 8.7 years, 72 patients experienced tumor recurrences; 18 (23.1%) in the TNBC group and 54 (11.3%) in the non-TNBC group (p=0.010), with cumulative 3-year rate of recurrence of 12.8% and 5.3%, respectively (p=0.010). Ten-year relapse-free survival (RFS; 75.6% vs. 87.5%, p=0.004) and overall survival (OS; 83.0% vs. 91.4%, p=0.002) rates were significantly lower in the TNBC group. Multivariate analysis showed that triple negativity and histologic grade were independent predictors of shorter RFS and OS.
CONCLUSION
TNBC had more aggressive clinicopathologic characteristics and was associated with poorer survival in patients with stage I breast cancer. More intensive adjuvant chemotherapy or a different therapeutic strategy targeting this population is warranted.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Evaluation of the Survival Benefit of Different Chemotherapy Regimens in Patients with T1-2N0 Triple-Negative Breast Cancer
Hyun-Ah Kim, Min-Ki Seong, Eun-kyu Kim, Eunyoung Kang, Seho Park, Min Hee Hur, Byung Joo Song, Woo Chul Noh,
J Breast Cancer. 2015;18(3):271-278. doi: 10.4048/jbc.2015.18.3.271.Predictive Value of Molecular Subtyping for Locoregional Recurrence in Early-Stage Breast Cancer with N1 without Postmastectomy Radiotherapy
Ge Wen, Jin-Shan Zhang, Yu-Jing Zhang, Yu-Jia Zhu, Xiao-Bo Huang, Xun-Xing Guan
J Breast Cancer. 2016;19(2):176-184. doi: 10.4048/jbc.2016.19.2.176.
Reference
-
1. Wood WC, Muss HB, Solin LJ, Olopade OI. DeVita VT, Hellman S, Rosenberg SA, editors. Malignant tumors of the breast. Cancer: Principles and Practice of Oncology. 2005. 7th ed. Philadelphia: Lippincott Williams & Wilkins;610–615.2. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triplenegative phenotype: a population-based study from the California cancer Registry. Cancer. 2007. 109:1721–1728.
Article3. Allred DC, Clark GM, Tandon AK, Molina R, Tormey DC, Osborne CK, et al. HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol. 1992. 10:599–605.
Article4. Le Doussal V, Tubiana-Hulin M, Friedman S, Hacene K, Spyratos F, Brunet M. Prognostic value of histologic grade nuclear components of Scarff-Bloom-Richardson (SBR). An improved score modification based on a multivariate analysis of 1262 invasive ductal breast carcinomas. Cancer. 1989. 64:1914–1921.
Article5. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005. 353:1659–1672.6. Dahabreh IJ, Linardou H, Siannis F, Fountzilas G, Murray S. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. 2008. 13:620–630.
Article7. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005. 353:1673–1684.
Article8. Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006. 354:809–820.
Article9. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006. 295:2492–2502.
Article10. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007. 13(15 Pt 1):4429–4434.
Article11. Gerson R, Alban F, Villalobos A, Serrano A. Recurrence and survival rates among early breast cancer cases with triple negative immunophenotype. Gac Med Mex. 2008. 144:27–34.12. Kaplan HG, Malmgren JA, Atwood M. T1N0 triple negative breast cancer: risk of recurrence and adjuvant chemotherapy. Breast J. 2009. 15:454–460.
Article13. Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009. 15:593–602.
Article14. Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer. 2007. 7:203.
Article15. Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006. 24:5652–5657.
Article16. Rhee J, Han SW, Oh DY, Kim JH, Im SA, Han W, et al. The clinicopathologic characteristics and prognostic significance of triple-negativity in node-negative breast cancer. BMC Cancer. 2008. 8:307.
Article17. Uhm JE, Park YH, Yi SY, Cho EY, Choi YL, Lee SJ, et al. Treatment outcomes and clinicopathologic characteristics of triple-negative breast cancer patients who received platinum-containing chemotherapy. Int J Cancer. 2009. 124:1457–1462.
Article18. Hines SL, Vallow LA, Tan WW, McNeil RB, Perez EA, Jain A. Clinical outcomes after a diagnosis of brain metastases in patients with estrogenand/ or human epidermal growth factor receptor 2-positive versus triplenegative breast cancer. Ann Oncol. 2008. 19:1561–1565.
Article19. Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008. 26:1275–1281.
Article20. Heitz F, Harter P, Lueck HJ, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009. 45:2792–2798.
Article21. Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008. 68:3108–3114.
Article22. Ahn JS, Cho J, Kwon SY, Kang SH. Clinicopathologic characteristics and prognosis of early stage triple negative breast cancer: comparison with non-triple negative group. J Korean Surg Soc. 2009. 77:37–42.
Article23. Noh JM, Choi DH, Huh SJ, Park W, Yang JH, Nam SJ, et al. Patterns of recurrence after breast-conserving treatment for early stage breast cancer by molecular subtype. J Breast Cancer. 2011. 14:46–51.
Article24. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007. 357:2666–2676.
Article25. Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005. 11:5175–5180.
Article26. Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nat Rev Cancer. 2004. 4:814–819.
Article27. Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli ML, et al. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer. 2003. 88:1285–1291.
Article28. Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 2010. 28:1145–1153.
Article29. Alli E, Sharma VB, Sunderesakumar P, Ford JM. Defective repair of oxidative dna damage in triple-negative breast cancer confers sensitivity to inhibition of poly(ADP-ribose) polymerase. Cancer Res. 2009. 69:3589–3596.
Article30. O'Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011. 364:205–214.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comment to “Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosisâ€
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Treatment Outcomes of Weakly Positive Hormone Receptor Breast Cancer and Triple-Negative Breast Cancer
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Fear of Cancer Recurrence and Unmet Needs in Triple Negative Breast Cancer Survivors