J Breast Cancer.
2012 Jun;15(2):181-188. 10.4048/jbc.2012.15.2.181.
The Role of Neutrophil Estrogen Receptor Status on Maspin Synthesis via Nitric Oxide Production in Human Breast Cancer
- Affiliations
-
- 1Sinha Institute of Medical Science & Technology, Garia, India. asruksinha@yahoo.com
- 2Department of Chemistry, Jadavpur University, Kolkata, India.
- KMID: 2242188
- DOI: http://doi.org/10.4048/jbc.2012.15.2.181
Abstract
- PURPOSE
Estrogen, through its binding to nuclear estrogen receptor (ER), has been implicated in the development of human breast cancer. The presence or absence of ER in breast lesions has been used to classify breast cancer into ER+ or ER- type. Maspin, an anti-breast cancer protein produced in normal mammary cells, has also been reported to control the condition. Studies have been conducted to determine the role of ER+ and ER- status in neutrophils in the synthesis of maspin in human breast cancer.
METHODS
Maspin presence was determined by enzyme linked immunosorbent assay, while nitric oxide (NO) level was determined using the methemoglobin method.
RESULTS
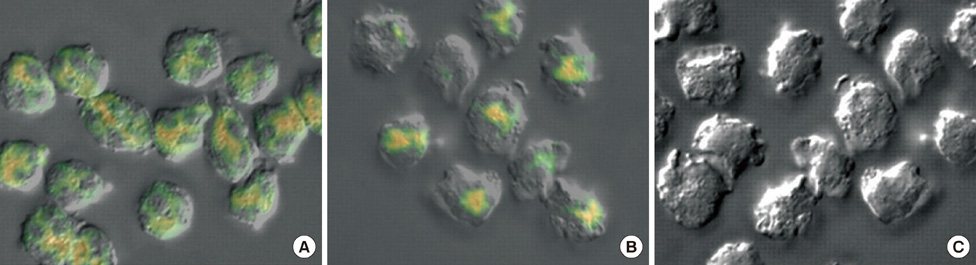
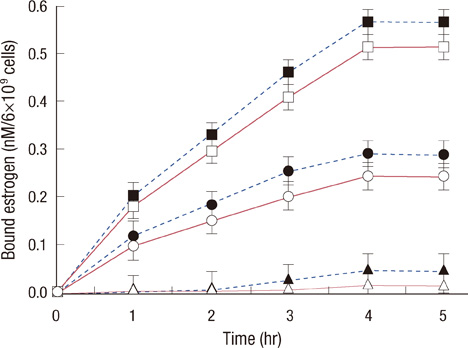
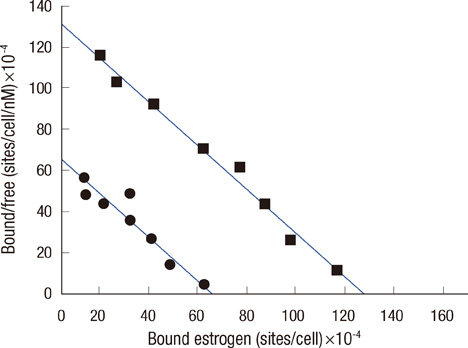
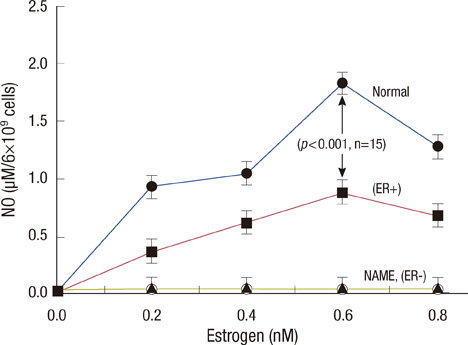
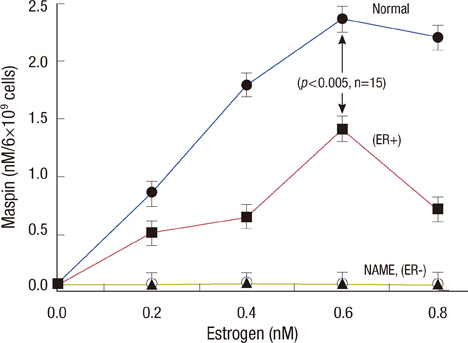
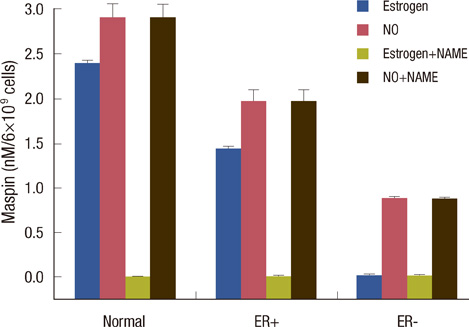
Scatchard plots of the equilibrium binding of estrogen demonstrated the presence of 4.18x10(7) receptors per normal neutrophil and 2.46x10(7) receptors per ER+ neutrophil with a similar dissociation constant (0.926 nM). The ER- type showed nonspecific estrogen binding only. At 0.6 nM estrogen, NO synthesis was maximally increased to 1.829 and 0.887 microM NO/10(9) cells at 4 hours in normal and ER+ neutrophils respectively, with synthesis of 2.383 and 1.422 nM maspin in normal and ER+ neutrophils respectively. Estrogen failed to produce these effects in ER- neutrophils.
CONCLUSION
ER status in neutrophils determined maspin synthesis in breast cancer through the stimulation of NO synthesis. Neutrophils with ER- status which do not produce any maspin when treated with estrogen, might imply a worse prognostic outcome in ER- breast cancer due to the lack of anti-breast cancer protein synthesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Schneider HP, Jackisch C. Potential benefits of estrogens and progestogens on breast cancer. Int J Fertil Womens Med. 1998. 43:278–285.2. Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000. 6:225–236.
Article3. Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994. 263:526–529.
Article4. Hojo T, Akiyama Y, Nagasaki K, Maruyama K, Kikuchi K, Ikeda T, et al. Association of maspin expression with the malignancy grade and tumor vascularization in breast cancer tissues. Cancer Lett. 2001. 171:103–110.
Article5. Liu T, Pemberton PA, Robertson AD. Three-state unfolding and self-association of maspin, a tumor-suppressing serpin. J Biol Chem. 1999. 274:29628–29632.
Article6. Girish GV, Bhattacharya G, Sinha AK. The role of insulin dependent NO synthesis in the impaired production of maspin in human breast cancer. J Cancer Res Clin Oncol. 2006. 132:389–398.
Article7. Tlaskalová-Hogenová H, Stépánková R. Development of antibody formation in germ-free and conventionally reared rabbits: the role of intestinal lymphoid tissue in antibody formation to E. coli antigens. Folia Biol (Praha). 1980. 26:81–93.8. Cox RD, Frank CW. Determination of nitrate and nitrite in blood and urine by chemiluminescence. J Anal Toxicol. 1982. 6:148–152.
Article9. Klock JC, Bainton DF. Degranulation and abnormal bactericidal function of granulocytes procured by reversible adhesion to nylon wool. Blood. 1976. 48:149–161.
Article10. Cook L, Ross AM, Knight GB, Agnello V. Use of whole blood specimens for routine clinical quantitation of hepatitis C virus RNA does not increase assay sensitivity. J Clin Microbiol. 2000. 38:4326–4331.
Article11. Zimmerman R, Paluch U, Sprinzl M, Neupert W. Cell-free synthesis of the mitochondrial ADP/ATP carrier protein of Neurospora crassa. Eur J Biochem. 1979. 99:247–252.
Article12. Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972. 109:129–135.13. Motomura K, Ishitobi M, Komoike Y, Koyama H, Nagase H, Inaji H, et al. Expression of estrogen receptor beta and phosphorylation of estrogen receptor alpha serine 167 correlate with progression-free survival in patients with metastatic breast cancer treated with aromatase inhibitors. Oncology. 2010. 79:55–61.
Article14. Kahn NN, Sinha AK. Stimulation of prostaglandin E1 binding to human blood platelet membrane by insulin and the activation of adenylate cyclase. J Biol Chem. 1990. 265:4976–4981.
Article15. Scatchard G. The attractions of proteins for small molecules and ions. Ann N Y Acad Sci. 1949. 51:660–672.
Article16. Sakuma I, Stuehr DJ, Gross SS, Nathan C, Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988. 85:8664–8667.
Article17. Whiting KP, Restall CJ, Brain PF. Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sci. 2000. 67:743–757.
Article18. Mauvais-Jarvis P, Kuttenn F, Gompel A. Antiestrogen action of progesterone in breast tissue. Breast Cancer Res Treat. 1986. 8:179–188.
Article19. Kiba T, Inamoto T, Nishimura T, Ueno M, Yanagihara K, Teramukai S, et al. The reversal of recurrence hazard rate between ER positive and negative breast cancer patients with axillary lymph node dissection (pathological stage I-III) 3 years after surgery. BMC Cancer. 2008. 8:323.
Article20. Rochefort H, Glondu M, Sahla ME, Platet N, Garcia M. How to target estrogen receptor-negative breast cancer? Endocr Relat Cancer. 2003. 10:261–266.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathological Significance of Maspin Expression in Breast Cancer
- Effect of Erythropoietin on the Production of Nitric Oxide in Trabecular Meshwork Cells
- Nitric Oxide Production in Mouse's Microglial Cells by Human Chorionic Gonadotropin
- Expression of Cyclooxygenase-2 in Human Breast Carcinoma: Relevance to Tumor Angiogenesis and Expression of Estrogen Receptor
- The Potential Role of Nitric Oxide in Halting Cancer Progression Through Chemoprevention