J Breast Cancer.
2012 Jun;15(2):172-180. 10.4048/jbc.2012.15.2.172.
The Glycolytic Phenotype is Correlated with Aggressiveness and Poor Prognosis in Invasive Ductal Carcinomas
- Affiliations
-
- 1Department of Pathology, Hanyang University College of Medicine, Seoul, Korea. sspaik@hanyang.ac.kr
- 2Department of Surgery, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2242187
- DOI: http://doi.org/10.4048/jbc.2012.15.2.172
Abstract
- PURPOSE
Glucose uptake and glycolytic metabolism are enhanced in cancer cells, and increased expression of glucose transporter 1 (GLUT1) has also been reported. The aim of this study was to investigate GLUT1 expression in human breast tissues and invasive ductal carcinomas.
METHODS
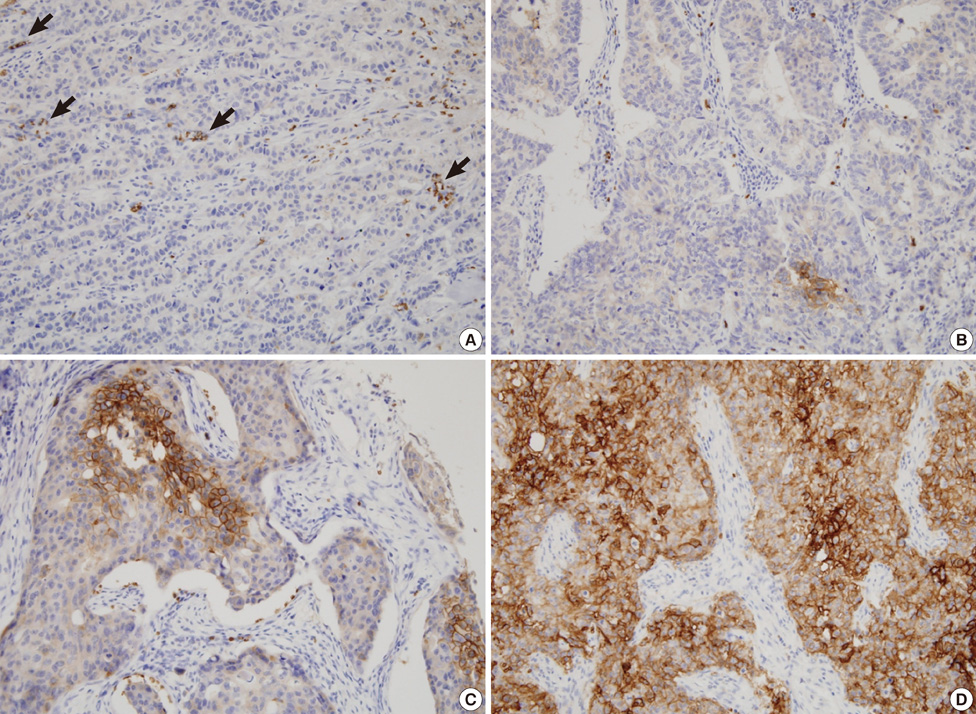
We used tissue microarrays consisting of normal breast tissue, ductal hyperplasia, ductal carcinoma in situ, invasive ductal carcinoma, and lymph node metastases. We examined GLUT1 expression in the microarrays by immunohistochemistry, reviewed the medical records and performed a clinicopathological analysis.
RESULTS
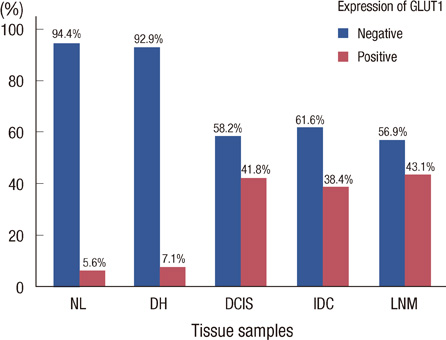
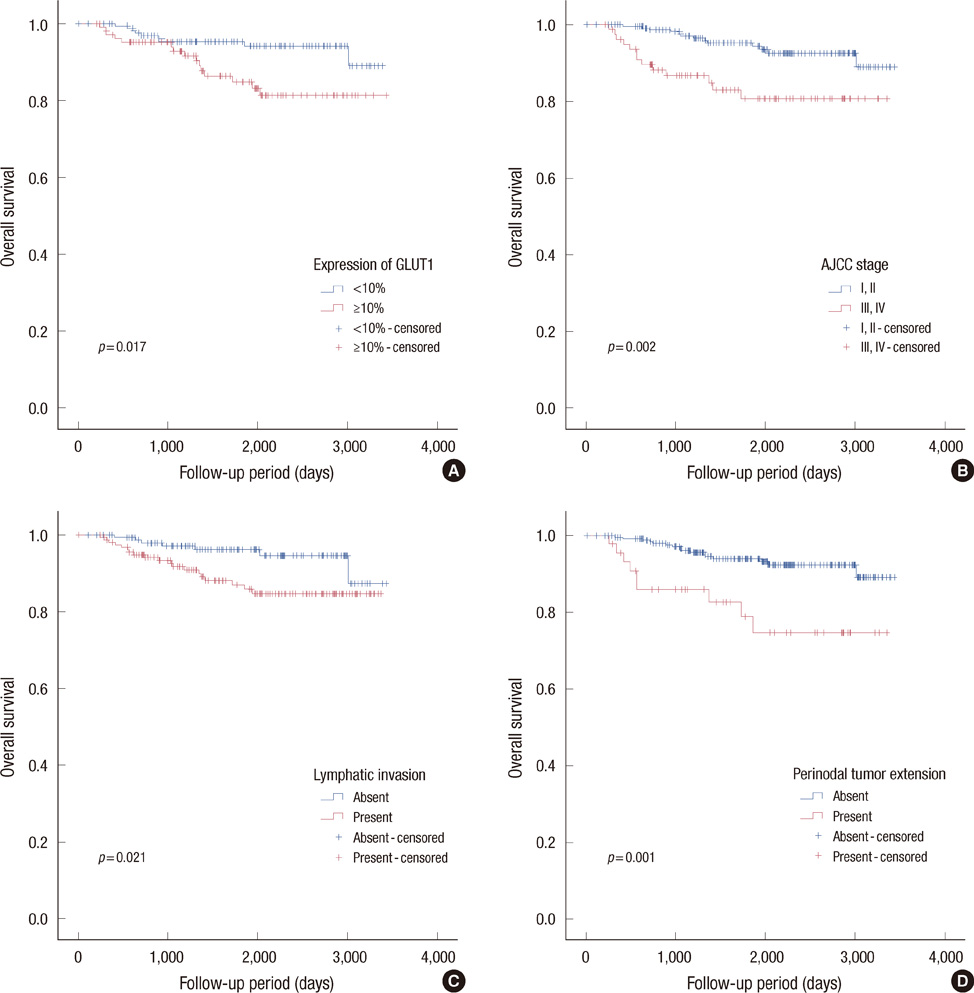
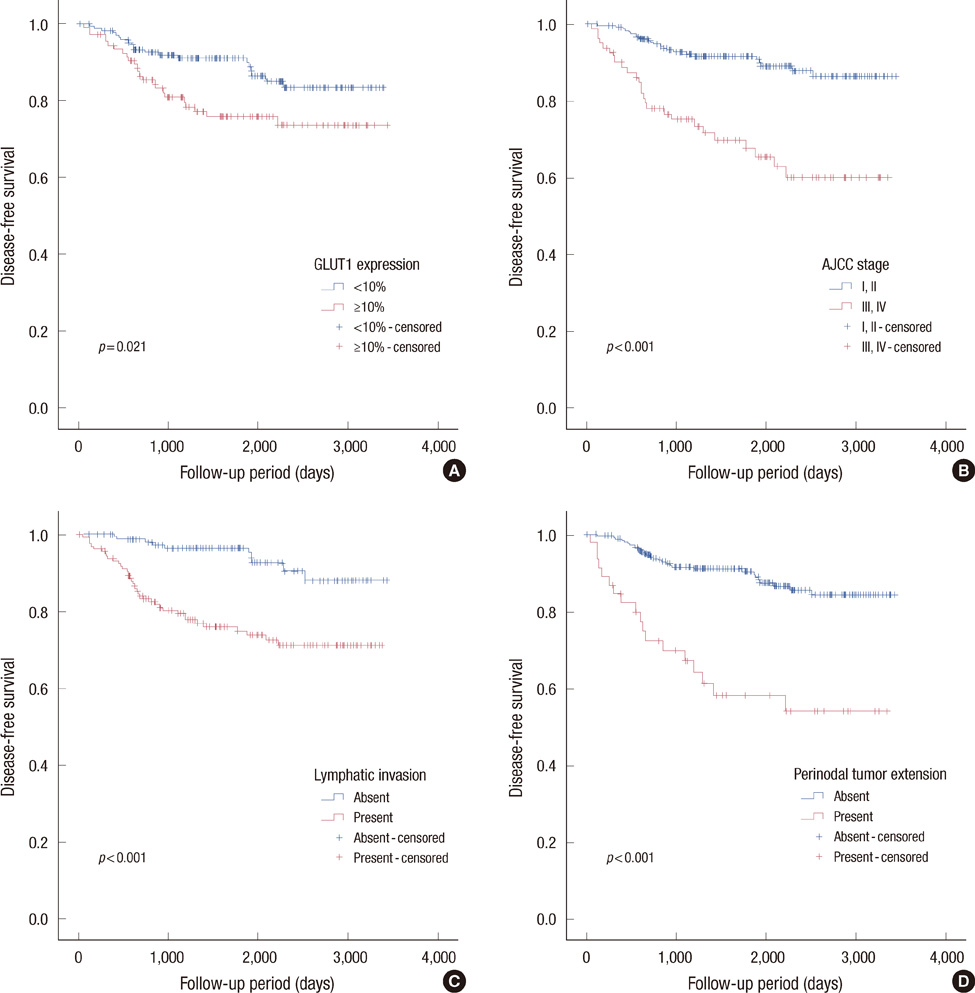
Membranous GLUT1 expression was observed in normal and tumor cells. GLUT1 expression was higher in ductal carcinoma in situ, invasive ductal carcinoma, and lymph node metastasis than in normal tissue and ductal hyperplasia (p=0.002). Of 276 invasive ductal carcinomas, 106 (38.4%) showed GLUT1 expression. GLUT1 expression was correlated with higher histologic grade (p<0.001), larger tumor size (p=0.025), absence of estrogen receptor (p<0.001), absence of progesterone receptor (p<0.001), and triple-negative phenotype (p<0.001). In univariate survival analysis, patients with GLUT1 expression had poorer overall survival and disease-free survival (p=0.017 and p=0.021, respectively, log-rank test). In multivariate survival analysis with the Cox proportional hazards model, GLUT1 expression was an independent prognostic factor of poorer overall survival and disease-free survival (p=0.017 and p=0.019, respectively).
CONCLUSION
GLUT1 expression seems to play an important role in malignant transformation, and the glycolytic phenotype in invasive ductal carcinoma may indicate aggressive biological behavior and a worse prognosis.
MeSH Terms
-
Breast
Carcinoma, Ductal
Carcinoma, Intraductal, Noninfiltrating
Disease-Free Survival
Estrogens
Glucose
Glucose Transport Proteins, Facilitative
Humans
Hyperplasia
Immunohistochemistry
Lymph Nodes
Medical Records
Neoplasm Metastasis
Phenotype
Prognosis
Proportional Hazards Models
Receptors, Progesterone
Estrogens
Glucose
Glucose Transport Proteins, Facilitative
Receptors, Progesterone
Figure
Cited by 1 articles
-
Clinicopathological Significance of Dual-Specificity Protein Phosphatase 4 Expression in Invasive Ductal Carcinoma of the Breast
Hyunsung Kim, Se Min Jang, Hyein Ahn, Jongmin Sim, Kijong Yi, Yumin Chung, Hulin Han, Abdul Rehman, Min Sung Chung, Kiseok Jang, Seung Sam Paik
J Breast Cancer. 2015;18(1):1-7. doi: 10.4048/jbc.2015.18.1.1.
Reference
-
1. Schmidt M, Voelker HU, Kapp M, Krockenberger M, Dietl J, Kammerer U. Glycolytic phenotype in breast cancer: activation of Akt, up-regulation of GLUT1, TKTL1 and down-regulation of M2PK. J Cancer Res Clin Oncol. 2010. 136:219–225.
Article2. Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin. 2006. 56:37–47.
Article3. Warburg O. On the origin of cancer cells. Science. 1956. 123:309–314.
Article4. Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003. 23:7315–7328.
Article5. Young CD, Lewis AS, Rudolph MC, Ruehle MD, Jackman MR, Yun UJ, et al. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS One. 2011. 6:e23205.
Article6. Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001. 276:9519–9525.7. Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009. 19:12–16.
Article8. Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007. 17:71–77.
Article9. Carvalho KC, Cunha IW, Rocha RM, Ayala FR, Cajaíba MM, Begnami MD, et al. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics (Sao Paulo). 2011. 66:965–972.
Article10. Park JM, Jung CK, Choi YJ, Lee KY, Kang JH, Kim MS, et al. The use of an immunohistochemical diagnostic panel to determine the primary site of cervical lymph node metastases of occult squamous cell carcinoma. Hum Pathol. 2010. 41:431–437.
Article11. Sakashita M, Aoyama N, Minami R, Maekawa S, Kuroda K, Shirasaka D, et al. Glut1 expression in T1 and T2 stage colorectal carcinomas: its relationship to clinicopathological features. Eur J Cancer. 2001. 37:204–209.12. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010. 134:907–922.
Article13. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007. 131:18–43.
Article14. Brown RS, Wahl RL. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer. 1993. 72:2979–2985.
Article15. Mellanen P, Minn H, Grénman R, Härkönen P. Expression of glucose transporters in head-and-neck tumors. Int J Cancer. 1994. 56:622–629.
Article16. Nagase Y, Takata K, Moriyama N, Aso Y, Murakami T, Hirano H. Immunohistochemical localization of glucose transporters in human renal cell carcinoma. J Urol. 1995. 153:798–801.
Article17. Merrall NW, Plevin R, Gould GW. Growth factors, mitogens, oncogenes and the regulation of glucose transport. Cell Signal. 1993. 5:667–675.
Article18. Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995. 36:1625–1632.19. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004. 4:891–899.
Article20. Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009. 121:29–40.
Article21. Zamora-León SP, Golde DW, Concha II, Rivas CI, Delgado-López F, Baselga J, et al. Expression of the fructose transporter GLUT5 in human breast cancer. Proc Natl Acad Sci U S A. 1996. 93:1847–1852.
Article22. Chen CL, Chu JS, Su WC, Huang SC, Lee WY. Hypoxia and metabolic phenotypes during breast carcinogenesis: expression of HIF-1alpha, GLUT1, and CAIX. Virchows Arch. 2010. 457:53–61.
Article23. Hao LS, Ni Q, Jia GQ, Wang G, Qian K, Liu YJ, et al. Expression of glucose transporter 1 in human breast carcinoma and its clinical significance. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009. 40:44–47.24. Kang SS, Chun YK, Hur MH, Lee HK, Kim YJ, Hong SR, et al. Clinical significance of glucose transporter 1 (GLUT1) expression in human breast carcinoma. Jpn J Cancer Res. 2002. 93:1123–1128.
Article25. Pinheiro C, Sousa B, Albergaria A, Paredes J, Dufloth R, Vieira D, et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol. 2011. 26:1279–1286.26. Ravazoula P, Batistatou A, Aletra C, Ladopoulos J, Kourounis G, Tzigounis B. Immunohistochemical expression of glucose transporter Glut1 and cyclin D1 in breast carcinomas with negative lymph nodes. Eur J Gynaecol Oncol. 2003. 24:544–546.27. Kim BJ, Cho SH, Jung GJ, Kim SS, Hong SH, Roh MS. Expression pattern of glucose transporter GLUT1 protein & its correlation with prognostic factors in breast cancer. J Korean Breast Cancer Soc. 2001. 4:161–166.
Article28. Ahn HJ, Lee KY, Lee SM, Koh SH, Hong SW, Oh SM, et al. Prognosis of GLUT1 expression in human breast carcinoma. J Korean Breast Cancer Soc. 2001. 4:167–171.
Article29. Kuo SJ, Wu YC, Chen CP, Tseng HS, Chen DR. Expression of glucose transporter-1 in Taiwanese patients with breast carcinoma: a preliminary report. Kaohsiung J Med Sci. 2006. 22:339–345.
Article30. Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995. 15(6B):2895–2898.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Survivin According to Malignant Progression of Breast Lesions
- Expression of MT-1 MMP, MMP2, MMP9 and TIMP2 mRNAs in Ductal Carcinoma in Situ and Invasive Ductal Carcinoma of the Breast
- Factors related with Axillary Lymph Nodes Metastases in T1 invasive ductal carcinomas of the Breast
- Associations between the Expression of Mucins (MUC1, MUC2, MUC5AC, and MUC6) and Clinicopathologic Parameters of Human Breast Ductal Carcinomas
- Expression of p21, p53 and bcl-2 Proteins in Invasive Ductal Carcinoma of the Breast