J Breast Cancer.
2013 Jun;16(2):152-158. 10.4048/jbc.2013.16.2.152.
Associations between the Expression of Mucins (MUC1, MUC2, MUC5AC, and MUC6) and Clinicopathologic Parameters of Human Breast Ductal Carcinomas
- Affiliations
-
- 1Department of Pathology, Breast and Thyroid Cancer Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. jhpath.sohn@samsung.com
- 2Department of Surgery, Breast and Thyroid Cancer Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2286394
- DOI: http://doi.org/10.4048/jbc.2013.16.2.152
Abstract
- PURPOSE
Mucins are members of the glycoprotein family expressed in benign and malignant epithelial cells. The aim of this study is to evaluate the relationships between the expression of mucins in breast ductal carcinoma and clinicopathologic parameters.
METHODS
We constructed tumor microarrays based on 240 cases of invasive ductal carcinoma and 40 cases of ductal carcinoma in situ (DCIS) using formalin fixed, paraffin embedded tissues. We examined the expressions of MUC1, MUC2, MUC5AC, and MUC6 by immunohistochemistry.
RESULTS
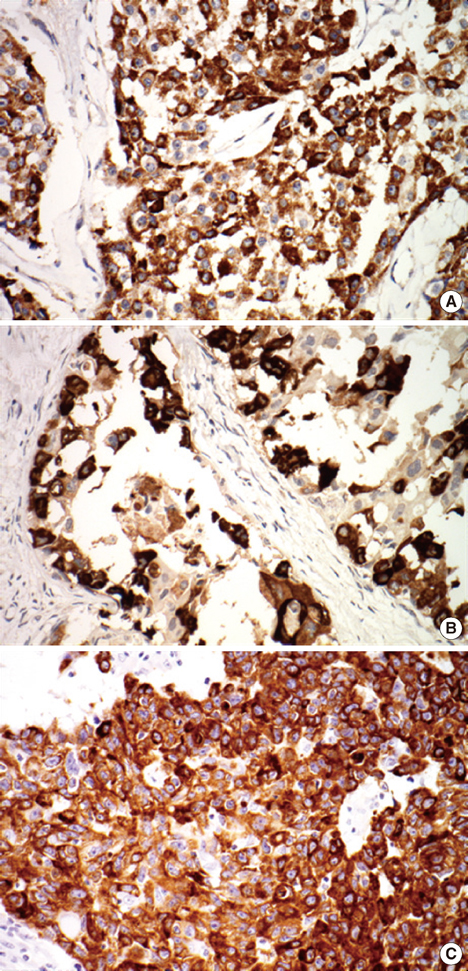
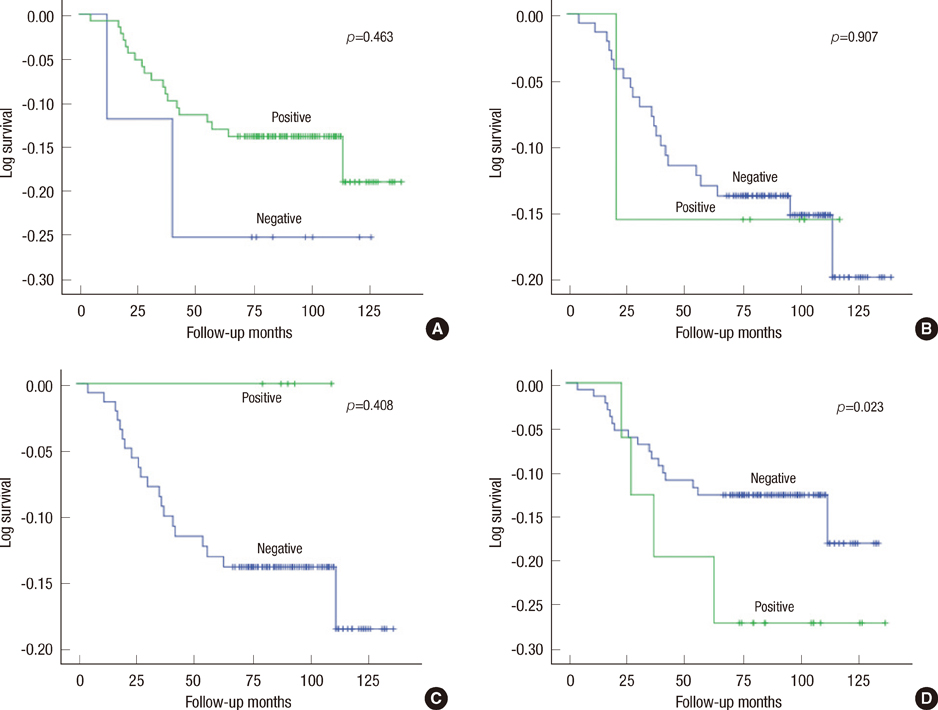
MUC1 demonstrated cytoplasmic, membranous, apical, and combinative expressions. Other mucins demonstrated cytoplasmic expression. In invasive ductal carcinoma, MUC1, MUC2, MUC5AC, and MUC6 were expressed in 93.6%, 6.2%, 4.8%, and 12.4% of cases, respectively; these rates were slightly, but not significantly, higher than observed in cases of DCIS. MUC1 expression was associated with estrogen receptor (ER) expression and negative MUC1 expression was associated with triple negativity. MUC6 expression was correlated with higher histologic grade, lymphatic invasion, lymph node metastasis, and HER2 positivity. No associations with any other clinicopathologic parameters were observed.
CONCLUSION
Most invasive ductal carcinomas of the breast express MUC1, and this expression is associated with ER expression. MUC6 expression is correlated with some clinicopathologic parameters that are indicators of poor prognosis. To evaluate the role of MUC6 as a potential biomarker, further studies are warranted.
MeSH Terms
Figure
Reference
-
1. Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995; 57:607–634.
Article2. Kim YS, Gum J Jr, Brockhausen I. Mucin glycoproteins in neoplasia. Glycoconj J. 1996; 13:693–707.
Article3. Fowler J, Vinall L, Swallow D. Polymorphism of the human muc genes. Front Biosci. 2001; 6:D1207–D1215.
Article4. Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004; 4:45–60.
Article5. Osako M, Yonezawa S, Siddiki B, Huang J, Ho JJ, Kim YS, et al. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993; 71:2191–2199.
Article6. Tashiro Y, Yonezawa S, Kim YS, Sato E. Immunohistochemical study of mucin carbohydrates and core proteins in human ovarian tumors. Hum Pathol. 1994; 25:364–372.
Article7. Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, et al. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998; 4:2605–2614.8. Yamashita K, Yonezawa S, Tanaka S, Shirahama H, Sakoda K, Imai K, et al. Immunohistochemical study of mucin carbohydrates and core proteins in hepatolithiasis and cholangiocarcinoma. Int J Cancer. 1993; 55:82–91.
Article9. Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, et al. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999; 49:45–54.
Article10. Patton S, Gendler SJ, Spicer AP. The epithelial mucin, MUC1, of milk, mammary gland and other tissues. Biochim Biophys Acta. 1995; 1241:407–423.
Article11. Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer. 2001; 91:1973–1982.
Article12. Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995; 129:255–265.
Article13. Kohlgraf KG, Gawron AJ, Higashi M, Meza JL, Burdick MD, Kitajima S, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003; 63:5011–5020.14. Gum JR Jr, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994; 269:2440–2446.
Article15. Chang SK, Dohrman AF, Basbaum CB, Ho SB, Tsuda T, Toribara NW, et al. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology. 1994; 107:28–36.
Article16. Adsay NV, Merati K, Nassar H, Shia J, Sarkar F, Pierson CR, et al. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol. 2003; 27:571–578.17. Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001; 25:26–42.
Article18. Matsukita S, Nomoto M, Kitajima S, Tanaka S, Goto M, Irimura T, et al. Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: comparison with invasive ductal carcinoma. Histopathology. 2003; 42:26–36.
Article19. Osunkoya AO, Adsay NV, Cohen C, Epstein JI, Smith SL. MUC2 expression in primary mucinous and nonmucinous adenocarcinoma of the prostate: an analysis of 50 cases on radical prostatectomy. Mod Pathol. 2008; 21:789–794.
Article20. De Bolos C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995; 109:723–734.
Article21. Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, et al. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995; 55:2681–2690.22. Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011; 24:157–167.
Article23. Chang E, Lee E, Yoo C, Oh SJ, Kim JS, Kang C. Cellular localization of MUC1 in benign and malignant breast lesions with the histological correlation and the prognostic significance. J Breast Cancer. 2005; 8:150–156.
Article24. Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, Paish EC, et al. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005; 18:1295–1304.
Article25. van der Vegt B, de Roos MA, Peterse JL, Patriarca C, Hilkens J, de Bock GH, et al. The expression pattern of MUC1 (EMA) is related to tumour characteristics and clinical outcome of invasive ductal breast carcinoma. Histopathology. 2007; 51:322–335.
Article26. Pereira MB, Dias AJ, Reis CA, Schmitt FC. Immunohistochemical study of the expression of MUC5AC and MUC6 in breast carcinomas and adjacent breast tissues. J Clin Pathol. 2001; 54:210–213.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of MUC Gene Proteins in Cholecystitis, Adenoma and Adenocarcinoma of the Gallbladder
- Mucin Expression According to the Progression of Gastric Carcinomas
- Clinicopathologic correlation with MUC expression in advanced gastric cancer
- Genetic Expression Pattern of Gastric Carcinomas According to Cellular Mucin Phenotypes
- Expression of MUC2 and MUC6 in Colorectal Adenomas and Adenocarcinomas