Korean Circ J.
2008 Nov;38(11):573-582. 10.4070/kcj.2008.38.11.573.
Transesophageal Echocardiographic Evaluation of Atherosclerosis
- Affiliations
-
- 1Division of Cardiology, Osaka Rosai Hospital, Osaka, Japan. mnishino@orh.go.jp
- KMID: 2225704
- DOI: http://doi.org/10.4070/kcj.2008.38.11.573
Abstract
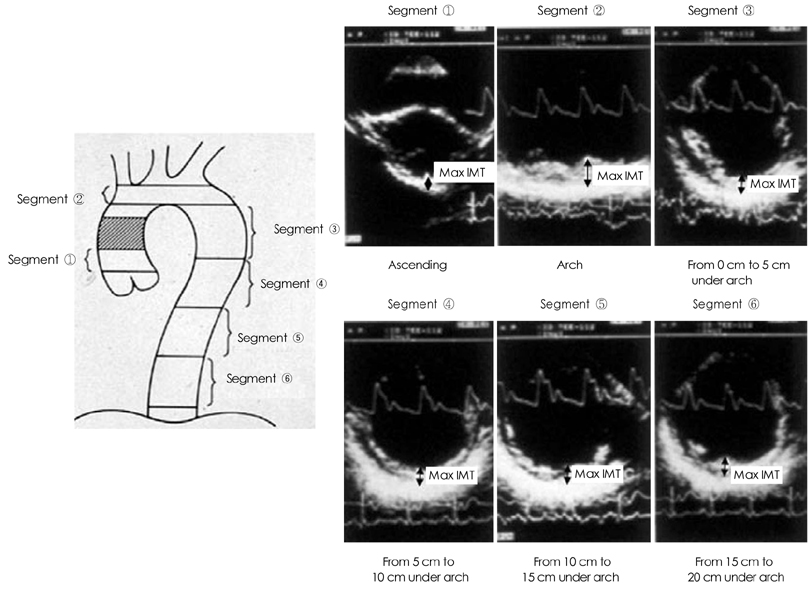
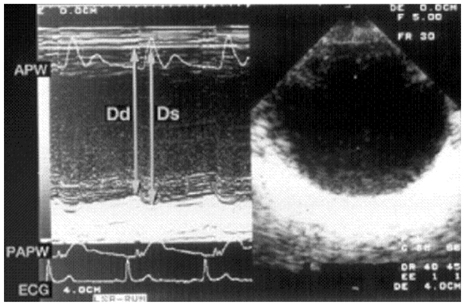
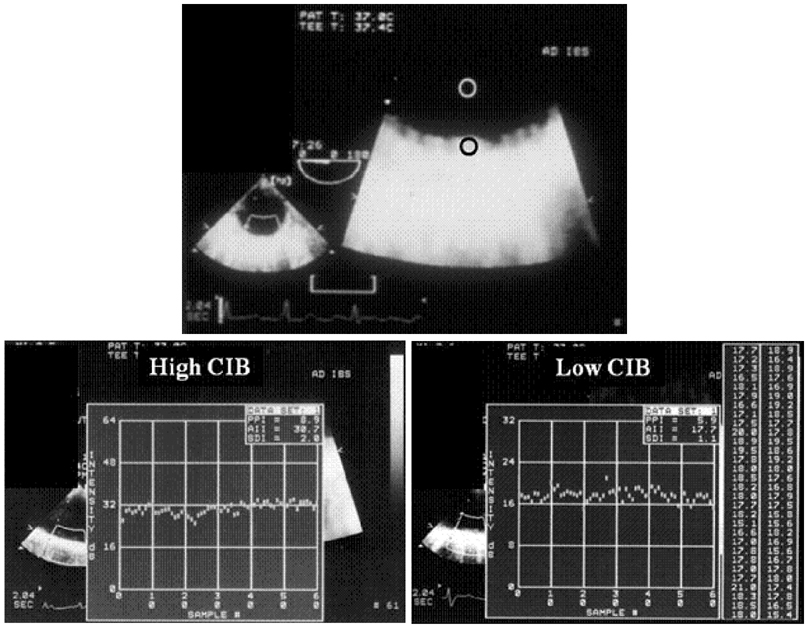
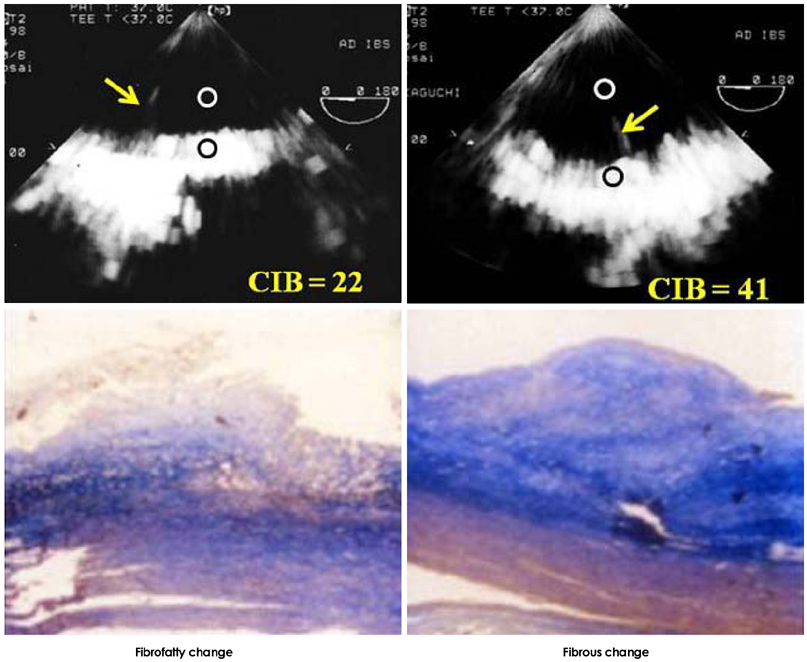
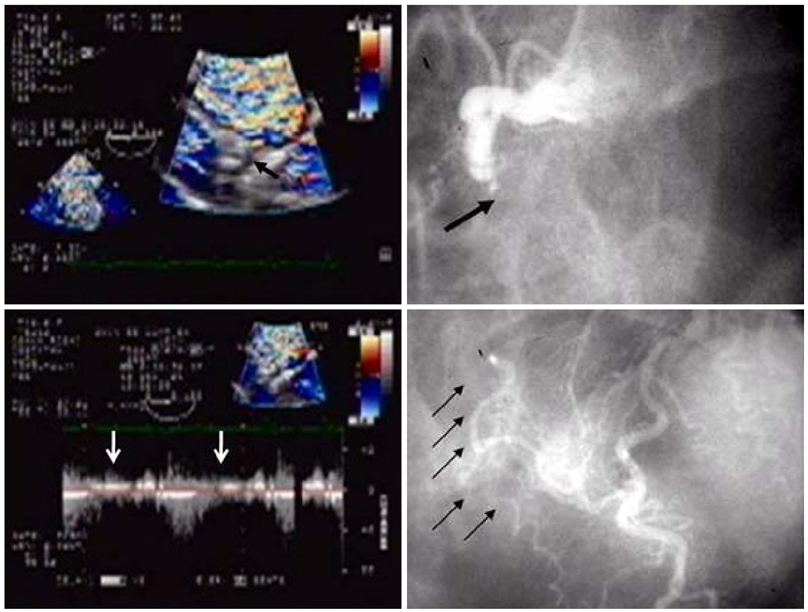
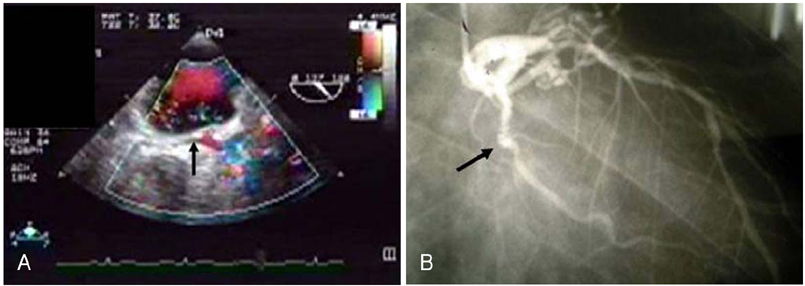
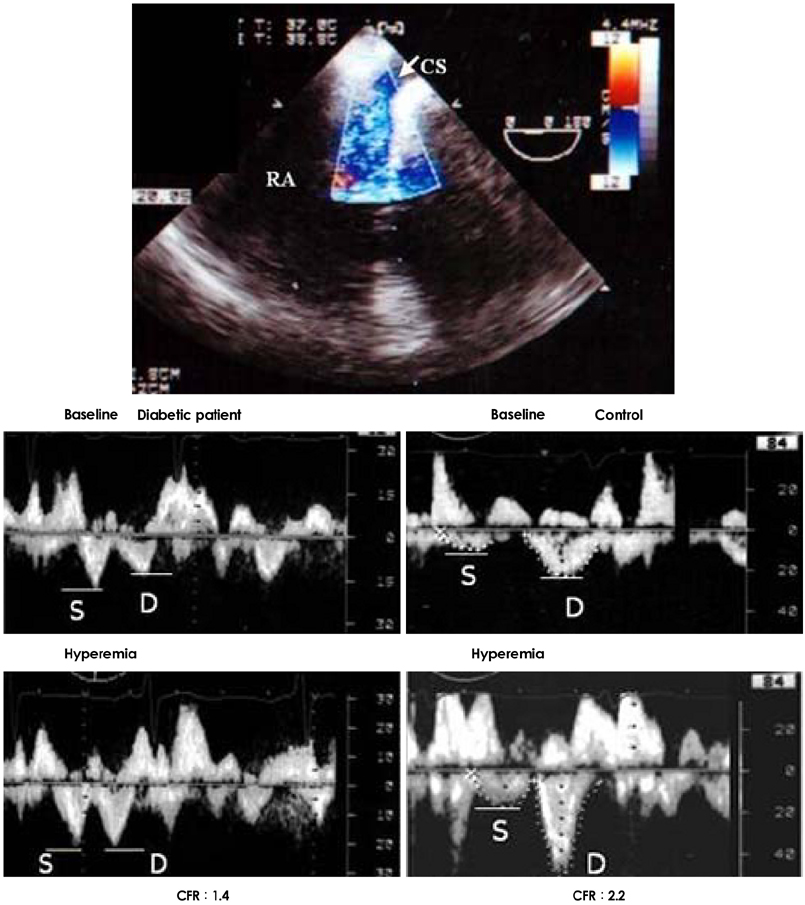
- Transesophageal echocardiography (TEE) is a promising method for evaluating thoracic aortic atherosclerosis and coronary atherosclerosis. The highest impact of TEE as a clinical tool is in searching for cardiac embolic sources in patients with stroke and atrial fibrillation and in conducting detailed evaluations in patients with valvular disease, especially those with mitral valvular disease. However, it is also clinically useful in the evaluation of thoracic aortic atherosclerosis and coronary atherosclerosis. TEE is capable of evaluating thoracic aortic atherosis (intima- media complex thickness) and sclerosis (stiffness parameter beta) simultaneously. In addition, TEE can evaluate coronary atherosclerosis by non-invasively revealing narrowing or occlusion of the coronary arteries and providing information about coronary flow reserve. TEE imaging has improved with the advent of harmonic imaging, multiplane probes, contrast agents, and three-dimensional TEE. Future technology, including integrated backscatter (IBS), tissue Doppler, and strain imaging, will lead to further improvements in TEE. Thoracic aortic atherosclerosis and coronary atherosclerosis assessment should be performed in any patient undergoing TEE.
MeSH Terms
Figure
Cited by 1 articles
-
Early Cardiac Valvular Changes in Ankylosing Spondylitis: A Transesophageal Echocardiography Study
So-Hee Park, Il-Suk Sohn, Byung-Hyun Joe, Hui-Jeong Hwang, Chang-Bum Park, Eun-Sun Jin, Jin-Man Cho, Chong-Jin Kim, Jong-Hoa Bae, Sang-Hoon Lee
J Cardiovasc Ultrasound. 2012;20(1):30-36. doi: 10.4250/jcu.2012.20.1.30.
Reference
-
1. Fazio GP, Redberg RF, Winslow T, Schiller NB. Transesophageal echocardiographically detected atherosclerotic aortic plaque is a marker for coronary artery disease. J Am Coll Cardiol. 1993. 21:144–150.2. Di Tullio MR, Homma S, Jin Z, Sacco RL. Aortic atherosclerosis, hypercoagulability, and stroke the APRIS (Aortic Plaque and Risk of Ischemic Stroke) study. J Am Coll Cardiol. 2008. 52:855–861.3. Frogoudaki A, Barbetseas J, Aggeli C, et al. Thoracic aorta atherosclerosis burden index predicts coronary artery disease in patients undergoing transesophageal echocardiography. Atherosclerosis. 2008. 197:232–236.4. Nishino M, Masugata H, Yamada Y, Abe H, Hori M, Kamada T. Evaluation of thoracic aortic atherosclerosis by transesophageal echocardiography. Am Heart J. 1994. 127:336–344.5. Khatibzadeh M, Mitusch R, Stierle U, Gromoll B, Sheikhzadeh A. Aortic atherosclerotic plaques as a source of systemic embolism. J Am Coll Cardiol. 1996. 27:664–669.6. Blankenhorn DH, Kramsch DM. Reversal of atherosis and sclerosis: the two components of atherosclerosis. Circulation. 1989. 79:1–7.7. Yamagishi M, Yasu T, Ohara K, Kuro M, Miyatake K. Detection of coronary blood flow associated with left main coronary artery stenosis by transesophageal Doppler color flow echocardiography. J Am Coll Cardiol. 1991. 17:87–93.8. Redberg RF, Sobol Y, Chou TM, et al. Adenosine-induced coronary vasodilation during transesophageal Doppler echocardiography: rapid and safe measurement of coronary flow reserve ratio can predict significant left anterior descending coronary stenosis. Circulation. 1995. 92:190–196.9. Youn HJ, Foster E. Transesophageal echocardiography (TEE) in the evaluation of the coronary arteries. Cardiol Clin. 2000. 18:833–848.10. Zehetgruber M, Mundigler G, Christ G, et al. Estimation of coronary flow reserve by transesophageal coronary sinus Doppler measurements in patients with syndrome X and patients with significant left coronary artery disease. J Am Coll Cardiol. 1995. 25:1039–1045.11. Vrublevsky AV, Boshchenko AA, Karpov RS. Reduced coronary flow reserve in the coronary sinus is a predictor of hemodynamically significant stenoses of the left coronary artery territory. Eur J Echocardiogr. 2004. 5:294–303.12. Nishino M, Hoshida S, Egami Y, et al. Coronary flow reserve by contrast enhanced transesophageal coronary sinus Doppler measurements can evaluate diabetic microvascular dysfunction. Circ J. 2006. 70:1415–1420.13. Tunick PA, Kronzon I. Atheromas of the thoracic aorta: clinical and therapeutic update. J Am Coll Cardiol. 2000. 35:545–554.14. Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation. 2006. 114:63–75.15. van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001. 32:454–460.16. Harloff A, Strecker C, Reinhard M, et al. Combined measurement of carotid stiffness and intima-media thickness improves prediction of complex aortic plaques in patients with ischemic stroke. Stroke. 2006. 37:2708–2712.17. Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006. 113:2363–2372.18. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986. 74:1399–1406.19. Nishino M, Ito T, Yasuno M, et al. Serum lipoprotein (a) as a risk factor for thoracic aortic atherosclerosis in subjects aged > 40 years. Am J Cardiol. 1993. 72:227–229.20. Tomochika Y, Okuda F, Tanaka N, et al. Improvement of atherosclerosis and stiffness of the thoracic descending aorta with cholesterol-lowering therapies in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1996. 16:955–962.21. Matsuzaki M, Hiramori K, Imaizumi T, et al. Intravascular ultrasound evaluation of coronary plaque regression by low density lipoprotein-apheresis in familial hypercholesterolemia: the Low Density Lipoprotein-Apheresis Coronary Morphology and Reserve Trial (LACMART). J Am Coll Cardiol. 2002. 40:220–227.22. Okazaki S, Yokoyama T, Miyauchi K, et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation. 2004. 110:1061–1068.23. Takayama T, Hiro T, Yamagishi M, et al. Rationale and design for a study using intravascular ultrasound to evaluate effects of rosuvastatin on coronary artery atheroma in Japanese subjects: COSMOS study (Coronary Atherosclerosis Study Measuring Effects of Rosuvastatin Using Intravascular Ultrasound in Japanese Subjects). Circ J. 2007. 71:271–275.24. McLean JW, Tomlinson JE, Kuang WJ, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987. 330:132–137.25. Peltier M, Iannetta Peltier MC, Sarano ME, Lesbre JP, Colas JL, Tribouilloy CM. Elevated serum lipoprotein(a) level is an independent marker of severity of thoracic aortic atherosclerosis. Chest. 2002. 121:1589–1594.26. Cantin B, Gagnon F, Moorjani S, et al. Is lipoprotein(a) an independent risk factor for ischemic heart disease in men?: The Quebec Cardiovascular Study. J Am Coll Cardiol. 1998. 31:519–525.27. Nishino M, Malloy MJ, Naya-Vigne J, Russell J, Kane JP, Redberg RF. Lack of association of lipoprotein(a) levels with coronary calcium deposits in asymptomatic postmenopausal women. J Am Coll Cardiol. 2000. 35:314–320.28. Bennet A, Di Angelantonio E, Erqou S, et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008. 168:598–608.29. Tribouilloy C, Peltier M, Colas L, et al. Fibrinogen is an independent marker for thoracic aortic atherosclerosis. Am J Cardiol. 1998. 81:321–326.30. Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech. 1980. 13:175–184.31. Reneman RS, van Merode T, Hick P, Muytjens AM, Hoeks AP. Age-related changes in carotid artery wall properties in men. Ultrasound Med Biol. 1986. 12:465–471.32. Lacombe F, Dart A, Dewar E, Jennings G, Cameron J, Laufer E. Arterial elastic properties in man: a comparison of echo-Doppler indices of aortic stiffness. Eur Heart J. 1992. 13:1040–1045.33. Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction: a noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989. 80:78–86.34. Tomochika Y, Tanaka N, Ono S, et al. Assessment by transesophageal echography of atherosclerosis of the descending thoracic aorta in patients with hypercholesterolemia. Am J Cardiol. 1999. 83:703–709.35. Wickline SA, Verdonk ED, Wong AK, Shepard RK, Miller JG. Structural remodeling of human myocardial tissue after infarction: quantification with ultrasonic backscatter. Circulation. 1992. 85:259–268.36. Wickline SA, Shepard RK, Daugherty A. Quantitative ultrasonic characterization of lesion composition and remodeling in atherosclerotic rabbit aorta. Arterioscler Thromb. 1993. 13:1543–1550.37. Takiuchi S, Ito H, Iwakura K, et al. Ultrasonic tissue characterization predicts myocardial viability in early stage of reperfused acute myocardial infarction. Circulation. 1998. 97:356–362.38. Nishino M, Hoshida S, Egami Y, et al. Ultrasonic integrated backscatter discloses intramyocardial hemorrhage in patients with acute myocardial infarction. Echocardiography. 2007. 24:52–60.39. Takiuchi S, Rakugi H, Honda K, et al. Quantitative ultrasonic tissue characterization can identify high-risk atherosclerotic alteration in human carotid arteries. Circulation. 2000. 102:766–770.40. Katakami N, Yamasaki Y, Kosugi K, et al. Tissue characterization identifies subjects with high risk of cardiovascular diseases. Diabetes Res Clin Pract. 2004. 63:93–102.41. Klein AL, Murray RD, Black IW, et al. Integrated backscatter for quantification of left atrial spontaneous echo contrast. J Am Coll Cardiol. 1996. 28:222–231.42. Ito T, Suwa M, Nakamura T, Miyazaki S, Hirota Y, Kawamura K. Influence of warfarin therapy on left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. Am J Cardiol. 1999. 84:857–859. A843. Suwa M, Ito T, Kobashi A, et al. Myocardial integrated ultrasonic backscatter in patients with dilated cardiomyopathy: prediction of response to beta-blocker therapy. Am Heart J. 2000. 139:905–912.44. Vitarelli A, Conde Y, Cimino E, et al. Aortic wall mechanics in the Marfan syndrome assessed by transesophageal tissue Doppler echocardiography. Am J Cardiol. 2006. 97:571–577.45. Vitarelli A, Conde Y, Cimino E, et al. Assessment of ascending aorta distensibility after successful coarctation repair by strain Doppler echocardiography. J Am Soc Echocardiogr. 2008. 21:729–736.46. Zwicky P, Daniel WG, Mugge A, Lichtlen PR. Imaging of coronary arteries by color-coded transesophageal Doppler echocardiography. Am J Cardiol. 1988. 62:639–640.47. Pandian NG, Hsu TL, Schwartz SL, et al. Multiplane transesophageal echocardiography: imaging planes, echocardiographic anatomy, and clinical experience with a prototype phased array OmniPlane probe. Echocardiography. 1992. 9:649–666.48. Memmola C, Iliceto S, Rizzon P. Detection of proximal stenosis of left coronary artery by digital transesophageal echocardiography: feasibility, sensitivity, and specificity. J Am Soc Echocardiogr. 1993. 6:149–157.49. Iliceto S, Caiati C, Aragona P, Verde R, Schlief R, Rizzon P. Improved Doppler signal intensity in coronary arteries after intravenous peripheral injection of a lung-crossing contrast agent (SHU 508A). J Am Coll Cardiol. 1994. 23:184–190.50. Redberg RF. Coronary flow by transesophageal Doppler echocardiography: do saccharide-based contrast agents sweeten the pot? J Am Coll Cardiol. 1994. 23:191–193.51. Mulvagh SL, Foley DA, Aeschbacher BC, Klarich KK, Seward JB. Second harmonic imaging of an intravenously administered echocardiographic contrast agent: visualization of coronary arteries and measurement of coronary blood flow. J Am Coll Cardiol. 1996. 27:1519–1525.52. Fischer TA, Menzel T, Kolsch B, Kolfhaus B, Mohr-Kahaly S. Quantitative analysis of the left main coronary artery by 3D echocardiography: role, possibilities and limitations of noninvasive imaging of the left coronary artery. Z Kardiol. 2002. 91:33–39.53. Hozumi T, Yoshida K, Akasaka T, et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998. 32:1251–1259.54. Fujimoto K, Watanabe H, Hozumi T, et al. New noninvasive diagnosis of myocardial ischemia of the left circumflex coronary artery using coronary flow reserve measurement by transthoracic Doppler echocardiography: comparison with thallium-201 single photon emission computed tomography. J Cardiol. 2004. 43:109–116.55. Youn HJ, Foster E. Demonstration of coronary artery flow using transthoracic Doppler echocardiography. J Am Soc Echocardiogr. 2004. 17:178–185.56. Puma JA, Sketch MH Jr, Tcheng JE, et al. The natural history of single-vessel chronic coronary occlusion: a 25-year experience. Am Heart J. 1997. 133:393–399.57. Bell MR, Berger PB, Bresnahan JF, Reeder GS, Bailey KR, Holmes DR Jr. Initial and long-term outcome of 354 patients after coronary balloon angioplasty of total coronary artery occlusions. Circulation. 1992. 85:1003–1011.58. Watanabe N, Akasaka T, Yamaura Y, et al. Noninvasive detection of total occlusion of the left anterior descending coronary artery with transthoracic Doppler echocardiography. J Am Coll Cardiol. 2001. 38:1328–1332.59. Hirata K, Watanabe H, Hozumi T, et al. Simple detection of occluded coronary artery using retrograde flow in septal branch and left anterior descending coronary artery by transthoracic Doppler echocardiography at rest. J Am Soc Echocardiogr. 2004. 17:108–113.60. Otsuka R, Watanabe H, Hirata K, et al. A novel technique to detect total occlusion in the right coronary artery using retrograde flow by transthoracic Doppler echocardiography. J Am Soc Echocardiogr. 2005. 18:704–709.61. Hutchinson SJ, Shen A, Soldo S, Hla A, Kawanishi DT, Chandraratna PA. Transesophageal assessment of coronary flow velocity reserve during "regular" and "high"-dose dipyridamole stress testing. Am J Cardiol. 1996. 77:1164–1168.62. Sonoda S, Takeuchi M, Nakashima Y, Kuroiwa A. Safety and optimal dose of intracoronary adenosine 5'-triphosphate for the measurement of coronary flow reserve. Am Heart J. 1998. 135:621–627.63. Matsumura Y, Hozumi T, Watanabe H, et al. Cut-off value of coronary flow velocity reserve by transthoracic Doppler echocardiography for diagnosis of significant left anterior descending artery stenosis in patients with coronary risk factors. Am J Cardiol. 2003. 92:1389–1393.64. Lethen H, P Tries H, Kersting S, Lambertz H. Validation of noninvasive assessment of coronary flow velocity reserve in the right coronary artery: a comparison of transthoracic echocardiographic results with intracoronary Doppler flow wire measurements. Eur Heart J. 2003. 24:1567–1575.65. Watanabe H, Hozumi T, Hirata K, et al. Noninvasive coronary flow velocity reserve measurement in the posterior descending coronary artery for detecting coronary stenosis in the right coronary artery using contrast-enhanced transthoracic Doppler echocardiography. Echocardiography. 2004. 21:225–233.66. Opherk D, Mall G, Zebe H, Schwarz F, Weihe E, Manthey J, et al. Reduction of coronary reserve: a mechanism for angina pectoris in patients with arterial hypertension and normal coronary arteries. Circulation. 1984. 69:1–7.67. Cannon RO 3rd, Watson RM, Rosing DR, Epstein SE. Angina caused by reduced vasodilator reserve of the small coronary arteries. J Am Coll Cardiol. 1983. 1:1359–1373.68. Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL. Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med. 1982. 307:1362–1366.69. Youn HJ, Park CS, Cho EJ, et al. Left bundle branch block disturbs left anterior descending coronary artery flow: study using transthoracic Doppler echocardiography. J Am Soc Echocardiogr. 2005. 18:1093–1098.70. Youn HJ, Park CS, Cho EJ, et al. Pattern of exercise-induced ST change is related to coronary flow reserve in patients with chest pain and normal coronary angiogram. Int J Cardiol. 2005. 101:299–304.71. Kim HK, Kim YJ, Sohn DW, Park YB, Choi YS. Transthoracic echocardiographic evaluation of coronary flow reserve in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2004. 94:167–171.72. Sugioka K, Hozumi T, Takemoto Y, et al. Relation of early improvement in coronary flow reserve to late recovery of left ventricular function after beta-blocker therapy in patients with idiopathic dilated cardiomyopathy. Am Heart J. 2007. 153:1080e1–e6.73. Meimoun P, Malaquin D, Sayah S, et al. The coronary flow reserve is transiently impaired in tako-tsubo cardiomyopathy: a prospective study using serial Doppler transthoracic echocardiography. J Am Soc Echocardiogr. 2008. 21:72–77.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk Factor Analysis in Patients with Recurrent Cerebral Infarction by Transesophageal Echocardiography
- Carotid Atherosclerosis as a Marker of Atherosclerosis of the Thoracic Aorta in the Elderly
- A Case of Complete Papillary Muscle Rupture after Blunt Chest Trauma Confirmed by Transesophageal Echocardiography
- Prevalence of the patent foramen ovale in young patients with ischemic cerebrovascular disease: Transesophageal contrast echocardiographic study
- Echocardiographic detection of left atrial mobile calcium debris of trido valve surgery: a case report