Korean Circ J.
2010 Jul;40(7):321-327. 10.4070/kcj.2010.40.7.321.
Celecoxib Does Not Attenuate the Antiplatelet Effects of Aspirin and Clopidogrel in Healthy Volunteers
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. hyosoo@snu.ac.kr
- 2Cardiovascular Center, Seoul National University Hospital, Seoul, Korea.
- 3Cardiovascular Center, Bundang Seoul National University Hospital, Seongnam, Korea.
- KMID: 2225175
- DOI: http://doi.org/10.4070/kcj.2010.40.7.321
Abstract
- BACKGROUND AND OBJECTIVES
The prevalence of arthritis, which is often treated with celecoxib, is high in patients with coronary artery disease. Furthermore, celecoxib has been reported to reduce restenosis after coronary stenting by inhibiting expression of the proto-oncogene Akt. A concern is that celecoxib increases thrombogenicity by inhibiting the synthesis of prostacyclin in endothelial cells. However, it is not known whether the administration of celecoxib will attenuate the antiplatelet effects of aspirin and clopidogrel, which are used after stenting. We addressed this gap in our knowledge.
SUBJECTS AND METHODS
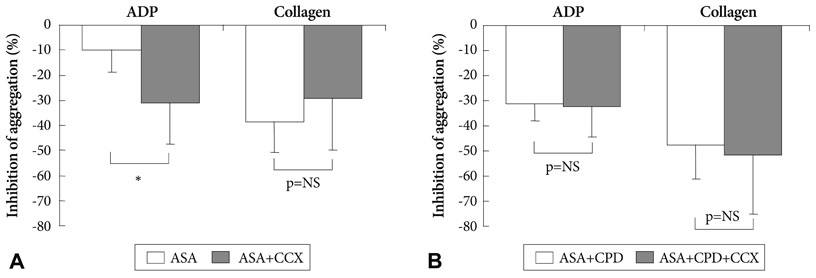
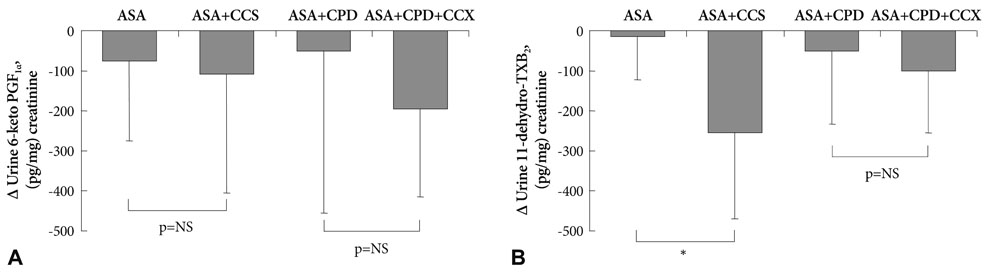
We recruited healthy volunteers (n=40) and randomized them into five subgroups (n=8 for each group: aspirin, celecoxib, aspirin+celecoxib, aspirin+clopidogrel, and aspirin+clopidogrel+celecoxib). Each subject received their medications for 6 days and blood samples were taken on day 0 and day 7. Celecoxib (200 mg twice a day), and/or aspirin (100 mg daily), and/or clopidogrel (75 mg daily) were administered. We compared platelet function among subgroups using light transmittance aggregometry and arachidonic acid metabolite assays.
RESULTS
Celecoxib treatment alone did not significantly affect platelet aggregation. The reduction in adenosine diphosphase (ADP)-induced platelet aggregation by aspirin+clopidogrel was not affected by addition of celecoxib (31.3+/-6.9% vs. 32.4+/-12.2%, p=0.83). Inhibition of collagen-induced platelet aggregation by aspirin+clopidogrel was not affected by addition of celecoxib (47.6+/-13.4% vs. 51.6+/-3.7%, p=0.69). Drug-induced changes in prostacyclin and thromboxane levels did not differ among treatment groups.
CONCLUSION
Celecoxib treatment does not interfere with the antiplatelet effects of aspirin or clopidogrel, suggesting that celecoxib can be safely administered in combination with dual antiplatelet therapy in patients with coronary stenting without increased thrombogenicity.
MeSH Terms
-
Adenosine
Arachidonic Acid
Arthritis
Aspirin
Blood Platelets
Coronary Artery Disease
Endothelial Cells
Epoprostenol
Humans
Light
Platelet Aggregation
Platelet Aggregation Inhibitors
Prevalence
Proto-Oncogenes
Pyrazoles
Stents
Sulfonamides
Thrombosis
Celecoxib
Ticlopidine
Adenosine
Arachidonic Acid
Aspirin
Epoprostenol
Platelet Aggregation Inhibitors
Pyrazoles
Sulfonamides
Ticlopidine
Figure
Cited by 1 articles
-
Strategies for the safe use of non-steroidal anti-inflammatory drugs
Ga Young Ahn, Sang-Cheol Bae
J Korean Med Assoc. 2018;61(6):367-375. doi: 10.5124/jkma.2018.61.6.367.
Reference
-
1. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000. 284:1247–1255.2. Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000. 342:1946–1952.3. Koo BK, Kim YS, Park KW, et al. Effect of celecoxib on restenosis after coronary angioplasty with a Taxus stent (COREA-TAXUS trial): an open-label randomised controlled study. Lancet. 2007. 370:567–574.4. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005. 352:1092–1102.5. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005. 352:1071–1080.6. Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004. 351:1709–1711.7. Hawk E, Viner JL. The adenoma prevention with celecoxib and prevention of colorectal sporadic adenomatous polyps trials: stepping stones to progress. Cancer Epidemiol Biomarkers Prev. 2007. 16:185–187.8. ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT). PLoS Clin Trials. 2006. 1:e33.9. Chae SC. Antiplatelet agents in high-risk patients with coronary artery disease. Korean Circ J. 2004. 34:23–27.10. Kim SS, Jeong MH, Sim DS, et al. Very late thrombosis of a drug-eluting stent after discontinuation of dual antiplatelet therapy in a patient treated with both drug-eluting and bare-metal stents. Korean Circ J. 2009. 39:205–208.11. Park JC, Jeong MH, Kim JH, et al. Clinical characteristics of coronary interventions in old aged patients. Korean Circ J. 1998. 28:256–261.12. Fiorina P, Perseghin G, De Cobelli F, et al. Altered kidney graft high-energy phosphate metabolism in kidney-transplanted endstage renal disease type 1 diabetic patients: a cross-sectional analysis of the effect of kidney alone and kidney-pancreas transplantation. Diabetes Care. 2007. 30:597–603.13. McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999. 96:272–277.14. FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001. 345:433–442.15. Arrebola MM, De la Cruz JP, Villalobos MA, Pinacho A, Guerrero A, Sanchez de la Cuesta F. In vitro effects of clopidogrel on the platelet-subendothelium interaction, platelet thromboxane and endothelial prostacyclin production, and nitric oxide synthesis. J Cardiovasc Pharmacol. 2004. 43:74–82.16. Leese PT, Hubbard RC, Karim A, Isakson PC, Yu SS, Geis GS. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol. 2000. 40:124–132.17. Van Ryn J, Kink-Eiband M, Kuritsch I, et al. Meloxicam does not affect the antiplatelet effect of aspirin in healthy male and female volunteers. J Clin Pharmacol. 2004. 44:777–784.18. Wilner KD, Rushing M, Walden C, et al. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol. 2002. 42:1027–1030.19. Renda G, Tacconelli S, Capone ML, et al. Celecoxib, ibuprofen, and the antiplatelet effect of aspirin in patients with osteoarthritis and ischemic heart disease. Clin Pharmacol Ther. 2006. 80:264–274.20. Yang HM, Kim HS, Park KW, et al. Celecoxib, a cyclooxygenase-2 inhibitor, reduces neointimal hyperplasia through inhibition of Akt signaling. Circulation. 2004. 110:301–308.21. Wang K, Tarakji K, Zhou Z, et al. Celecoxib, a selective cyclooxygenase-2 inhibitor, decreases monocyte chemoattractant protein1 expression and neointimal hyperplasia in the rabbit atherosclerotic balloon injury model. J Cardiovasc Pharmacol. 2005. 45:61–67.22. Steffel J, Hermann M, Greutert H, et al. Celecoxib decreases endothelial tissue factor expression through inhibition of c-Jun terminal NH2 kinase phosphorylation. Circulation. 2005. 111:1685–1689.23. Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003. 102:449–461.24. Solomon SD, Wittes J, Finn PV, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008. 117:2104–2113.25. McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006. 296:1633–1644.26. White WB, West CR, Borer JS, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol. 2007. 99:91–98.27. Johnsen SP, Larsson H, Tarone RE, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med. 2005. 165:978–984.28. Belton O, Byrne D, Kearney D, Leahy A, Fitzgerald DJ. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation. 2000. 102:840–845.29. Kobayashi T, Tahara Y, Matsumoto M, et al. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004. 114:784–794.30. Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005. 112:759–770.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aspirin and Clopidogrel Resistance in Drug Eluting Stent Era
- Evaluation of Bleeding After Denal Extraction in Patients Taking Single Antiplatelet Treatment
- Variability of Platelet Reactivity on Antiplatelet Therapy in Neurointervention Procedure
- Hypersensitivity to ticagrelor and low response to clopidogrel: a case report
- Successful sequential desensitization in a patient with drug hypersensitivity to three kinds of antiplatelet agents