J Rheum Dis.
2012 Apr;19(2):100-103. 10.4078/jrd.2012.19.2.100.
A Case of Rituximab Use in Rheumatoid Arthritis Following Anti-TNF-Associated Tuberculosis
- Affiliations
-
- 1Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea. kiwonmoon@kangwon.ac.kr
- KMID: 2223108
- DOI: http://doi.org/10.4078/jrd.2012.19.2.100
Abstract
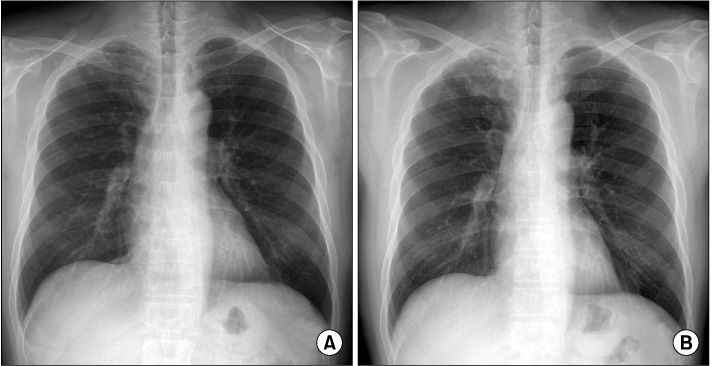
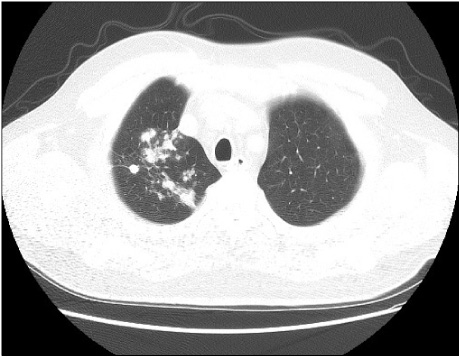
- Rituximab has been shown to be effective in rheumatoid arthritis (RA) and is recommended for patients exhibiting an inadequate response to tumor necrosis factor (TNF) inhibitors. To date, there have been no reports of tuberculosis in RA patients treated with rituximab. We report the use of rituximab in a TNF inhibitor-refractory RA patient who had developed tuberculosis. A 52-year-old man with RA had been treated with adalimumab for 3 months, but failed to respond well to the treatment. He reported fever, coughing, sputum, and weight loss. He was diagnosed with pulmonary tuberculosis and started anti-tuberculosis medication. His arthritis was not controlled for despite increasing the dose of prednisolone. He was treated with rituximab without serious adverse effects. Sixteen weeks later, he demonstrated improvement with both arthritis and tuberculosis.
Keyword
MeSH Terms
-
Antibodies, Monoclonal, Humanized
Antibodies, Monoclonal, Murine-Derived
Arthritis
Arthritis, Rheumatoid
Cough
Fever
Humans
Middle Aged
Prednisolone
Sputum
Tuberculosis
Tuberculosis, Pulmonary
Tumor Necrosis Factor-alpha
Weight Loss
Adalimumab
Rituximab
Antibodies, Monoclonal, Humanized
Antibodies, Monoclonal, Murine-Derived
Prednisolone
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Song JS. Review of tumor necrosis factor inhibitors on. Rheumatoid arthritis. J Korean Rheum Assoc. 2007. 14:1–14.2. Cush JJ. Safety overview of new disease-modifying antirheumatic drugs. Rheum Dis Clin North Am. 2004. 30:237–255.3. Edwards JC, Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford). 2001. 40:205–211.4. Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, et al. Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann Rheum Dis. 2011. 70:1575–1580.5. Hainsworth JD. Safety of rituximab in the treatment of B cell malignancies: implications for rheumatoid arthritis. Arthritis Res Ther. 2003. 5:Suppl 4. S12–S16.6. Buch MH, Smolen JS, Betteridge N, Breedveld FC, Burmester G, Dörner T, et al. Rituximab Consensus Expert Committee. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2011. 70:909–920.7. Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009. 68:25–32.8. Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004. 350:2572–2581.9. Kwok SK, Park SH. Guidelines for prevention of tuberculosis in patients with rheumatoid arthritis treated with TNF-α blockers. J Korean Rheum Assoc. 2007. 14:105–111.10. Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum. 2010. 39:327–346.11. Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007. 34:706–711.12. Burr ML, Malaviya AP, Gaston JH, Carmichael AJ, Ostör AJ. Rituximab in rheumatoid arthritis following anti-TNF-associated tuberculosis. Rheumatology (Oxford). 2008. 47:738–739.13. Jung N, Owczarczyk K, Hellmann M, Lehmann C, Fätkenheuer G, Hallek M, et al. Efficacy and safety of rituximab in a patient with active rheumatoid arthritis and chronic disseminated pulmonary aspergillosis and history of tuberculosis. Rheumatology (Oxford). 2008. 47:932–933.14. Vordermeier HM, Venkataprasad N, Harris DP, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996. 106:312–316.15. Lutt JR, Pisculli ML, Weinblatt ME, Deodhar A, Winthrop KL. Severe nontuberculous mycobacterial infection in 2 patients receiving rituximab for refractory myositis. J Rheumatol. 2008. 35:1683–1685.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rituximab for Rheumatoid Arthritis Following TNF-alpha Inhibitor Associated Splenic Tuberculosis
- New drugs for Rheumatoid arthritis
- Guidelines for Prevention of Tuberculosis in Patients with Rheumatoid Arthritis Treated with TNF-alpha Blockers
- Update on rheumatoid arthritis
- A Case of Tuberculous Arthritis Following the Use of Etanercept