J Rheum Dis.
2014 Feb;21(1):9-19. 10.4078/jrd.2014.21.1.9.
Dermatological Side Effects of Anti-tumor Necrosis Factor Alpha Therapy
- Affiliations
-
- 1Department of Dermatology, Hanyang University College of Medicine, Seoul, Korea. drko0303@hanyang.ac.kr
- KMID: 2222983
- DOI: http://doi.org/10.4078/jrd.2014.21.1.9
Abstract
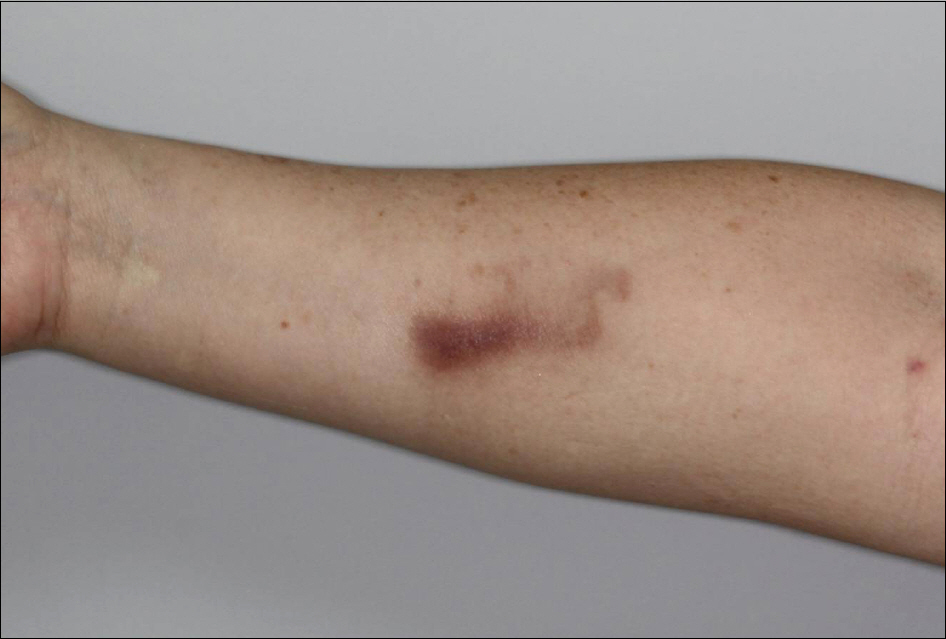
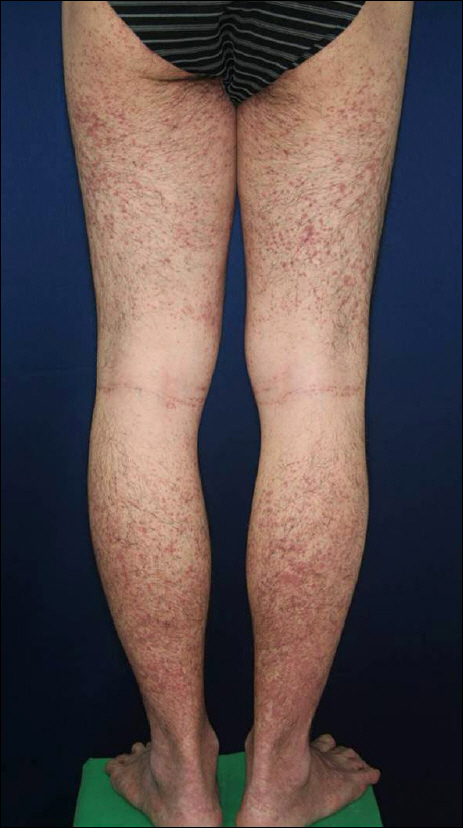
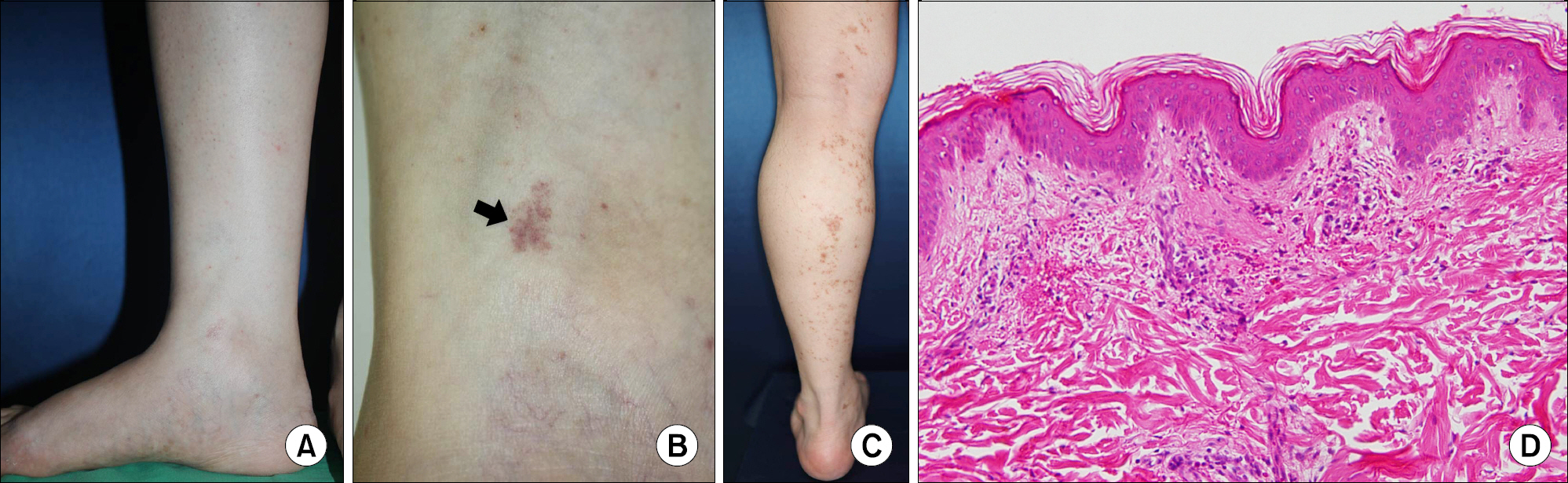
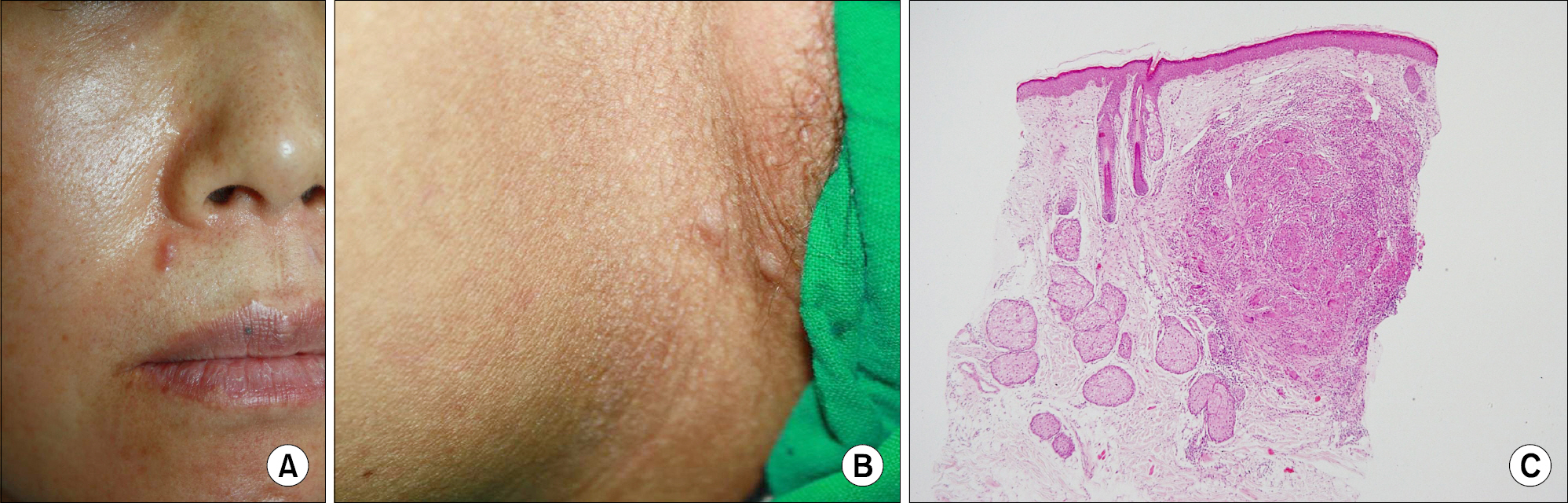
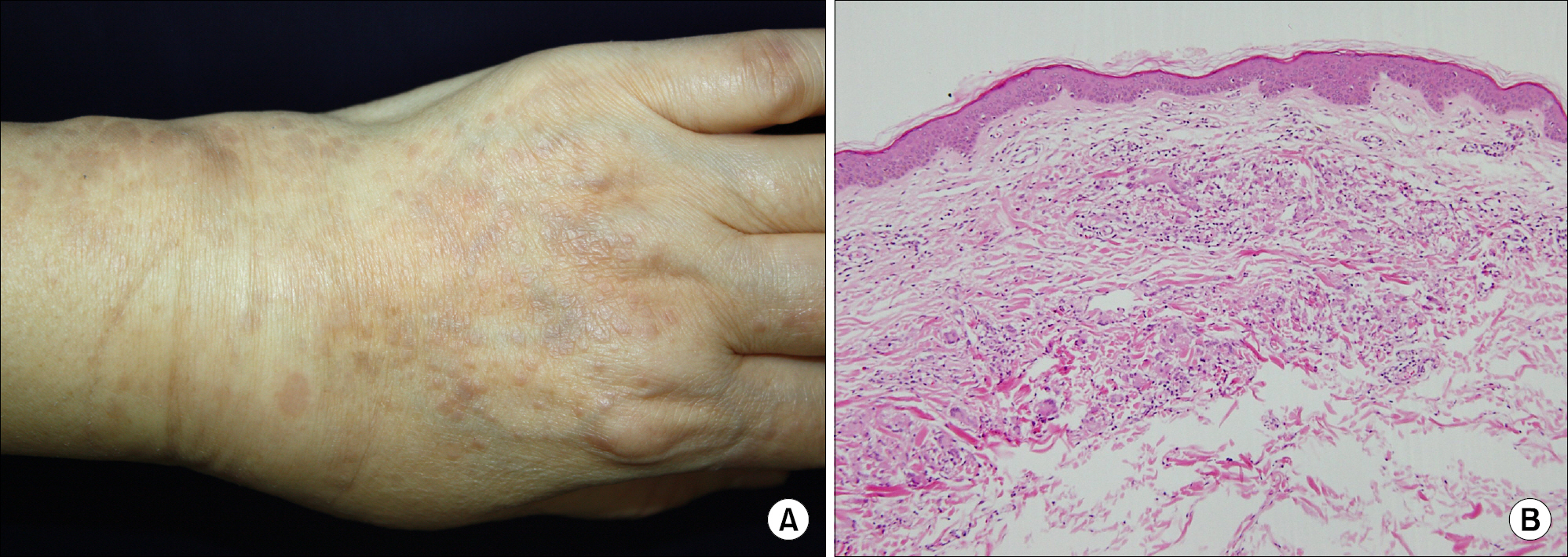
- As anti-tumor necrosis factor alpha (anti-TNFalpha) agents are progressively being used in various medical conditions, dermatological adverse events have been encountered more frequently. To understand such dermatological conditions that have been documented while undergoing anti-TNF therapy, we reviewed relevant literature, including case reports and case series. Reported dermatological conditions included infusion and injection site reaction, cutaneous infection, psoriasiform eruption, dermatitis, allergic rash, lupus-like lesion, vasculitis, lichenoid reaction, granulomatous reaction, hair loss, cutaneous infection, and cutaneous neoplasm. These events had varying strengths of causal association and severity therefore, drug discontinuation may or may not be required.
MeSH Terms
Figure
Reference
-
References
1. Moustou AE, Matekovits A, Dessinioti C, Antoniou C, Sfikakis PP, Stratigos AJ. Cutaneous side effects of antitumor necrosis factor biologic therapy: a clinical review. J Am Acad Dermatol. 2009; 61:486–504.
Article2. Hahn HJ, Jung JW, Park HJ, Lee YW, Choe YB, Ahn KJ. A case of psoriasiform dermatitis following adalimumab injection for treatment of ankylosing spondylitis. Korean J Dermatol. 2013; 51:743–5.3. Kim JY, Choi M, Cho KH. A case of pustular psoriasis developed during infliximab treatment for crohn's disease. Korean J Dermatol. 2012; 50:810–3.4. Park HY, Kim HS, Hong R. A case of bowen's disease during adalimumab treatment for Felty's syndrome. Korean J Med. 2012; 83:400–4.
Article5. Kim JH, Shin WU, Baek YS, Oh CH, Kim JH. Etanercept-induced Leukocytoclastic Vasculitis. Korean J Dermatol. 2012; 50:807–9.6. Jung WJ, Nam YJ, Lee SG, Kim JM, Song M, Kim MB, et al. Henoch-Schonlein purpura in a patient with ankylosing spondylitis after infliximab therapy. Korean J Med. 2013; 84:764–8.7. Lobel EZ, Korelitz BI, Warman JI. Red man syndrome and infliximab. J Clin Gastroenterol. 2003; 36:186.
Article8. Persley KM. Infliximab infusion reactions: desensitizing ourselves to the danger. Inflamm Bowel Dis. 2004; 10:62–3.
Article9. Baert F, Noman M, Vermeire S, Van Assche G, D' Haens G, Carbonez A, et al. Influence of immunogenicity on the longterm efficacy of infliximab in Crohn's disease. N Engl J Med. 2003; 348:601–8.
Article10. Han PD, Cohen RD. Managing immunogenic responses to infliximab: treatment implications for patients with Crohn's disease. Drugs. 2004; 64:1767–77.11. Baidoo L, Lichtenstein GR. What next after infliximab? Am J Gastroenterol. 2005; 100:80–3.
Article12. Werth VP, Levinson AI. Etanercept-induced injection site reactions: mechanistic insights from clinical findings and immunohistochemistry. Arch Dermatol. 2001; 137:953–5.13. Zeltser R, Valle L, Tanck C, Holyst MM, Ritchlin C, Gaspari AA. Clinical, histological, and immunophe-notypic characteristics of injection site reactions associated with etanercept: a recombinant tumor necrosis factor alpha receptor: Fc fusion protein. Arch Dermatol. 2001; 137:893–9.14. Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label ex-tension study. J Am Acad Dermatol. 2006; 55:598–606.
Article15. Gonzá lez-López MA, Martínez-Taboada VM, González-Vela MC, Blanco R, Fernández-Llaca H, Rodríguez-Valverde V, et al. Recall injection-site reactions associated with etanercept therapy: report of two new cases with immunohistochemical analysis. Clin Exp Dermatol. 2007; 32:672–4.
Article16. Wollina U, Hansel G, Koch A, Schönlebe J, Köstler E, Haroske G. Tumor necrosis factor-alpha inhibitor-induced psoriasis or psoriasiform exanthemata: first 120 cases from the literature including a series of six new patients. Am J Clin Dermatol. 2008; 9:1–14.17. Harrison MJ, Dixon WG, Watson KD, King Y, Groves R, Hyrich KL, et al. British Society for Rheumatology Biologics Register Control Centre Consortium; BSRBR. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2009; 68:209–15.18. Cohen JD, Bournerias I, Buffard V, Paufler A, Chevalier X, Bagot M, et al. Psoriasis induced by tumor necrosis factor-alpha antagonist therapy: a case series. J Rheumatol. 2007; 34:380–5.19. Nestle FO, Gilliet M. Defining upstream elements of psoriasis pathogenesis: an emerging role for interferon alpha. J Invest Dermatol. 2005; 125:xiv–xv.20. Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005; 102:3372–7.21. Verea MM, Del Pozo J, Yebra-Pimentel MT, Porta A, Fonseca E. Psoriasiform eruption induced by infliximab. Ann Pharmacother. 2004; 38:54–7.
Article22. Seneschal J, Lepreux S, Bouyssou-Gauthier ML, Heliot-Hosten I, Economou A, Dehais J, et al. Psoriasiform drug eruptions under anti-TNF treatment of arthritis are not true psoriasis. Acta Derm Venereol. 2007; 87:77–80.
Article23. Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by antitumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005; 52:2513–8.
Article24. Takahashi H, Hashimoto Y, Ishida-Yamamoto A, Ashida T, Kohgo Y, Iizuka H. Psoriasiform and pustular eruption induced by infliximab. J Dermatol. 2007; 34:468–72.
Article25. Flendrie M, Vissers WH, Creemers MC, de Jong EM, van de Kerkhof PC, van Riel PL. Dermatological conditions during TNF-alpha-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2005; 7:R666–76.26. Lee HH, Song IH, Friedrich M, Gauliard A, Detert J, Röwert J, et al. Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-alpha antagonists. Br J Dermatol. 2007; 156:486–91.27. Vestergaard C, Deleuran M, Kragballe K. Two cases of atopic dermatitis-like conditions induced in psoriasis patients treated with infliximab. J Eur Acad Dermatol Venereol. 2007; 21:1272–4.
Article28. Mangge H, Gindl S, Kenzian H, Schauenstein K. Atopic dermatitis as a side effect of antitumor necrosis factor-alpha therapy. J Rheumatol. 2003; 30:2506–7.29. Ladizinski B, Lee KC. Infliximab-Induced Urticaria. J Emerg Med. 2014; [Epub ahead of print].
Article30. Corominas H, Sánchez-Eslava L, García G, Padró I, Aimarich C, Gonzàlez J, et al. Safety profile of biological intravenous therapy in a rheumatoid arthritis patients cohort. Clinical nursing monitoring (Sebiol study). Reumatol Clin. 2013; 9:80–4.
Article31. Puxeddu I, Giori L, Rocchi V, Bazzichi L, Bombardieri S, Tavoni A, et al. Hypersensitivity reactions during treatment with infliximab, etanercept, and adalimumab. Ann Allergy Asthma Immunol. 2012; 108:123–4.
Article32. Habal F, Huang V. Angioedema associated with Crohn's disease: response to biologics. World J Gastroenterol. 2012; 18:4787–90.
Article33. Wilson LH, Eliason MJ, Leiferman KM, Hull CM, Powell DL. Treatment of refractory chronic urticaria with tumor necrosis factor-alfa inhibitors. J Am Acad Dermatol. 2011; 64:1221–2.
Article34. Atzeni F, Turiel M, Capsoni F, Doria A, Meroni P, Sarzi-Puttini P. Autoimmunity and anti-TNF-alpha agents. Ann N Y Acad Sci. 2005; 1051; 559–69.35. Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004; 51:534–42.
Article36. Poulalhon N, Begon E, Lebbé C, Lioté F, Lahfa M, Bengoufa D, et al. A followup study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007; 156:329–36.
Article37. De Bandt M, Sibilia J, Le Loët X, Prouzeau S, Fautrel B, Marcelli C, et al. Club Rhumatismes et Inflammation. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005; 7:R545–51.
Article38. Mohan N, Edwards ET, Cupps TR, Slifman N, Lee JH, Siegel JN, et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004; 31:1955–8.39. Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007; 86:242–51.40. Lebas D, Staumont-Sallé D, Solau-Gervais E, Flipo RM, Delaporte E. Cutaneous manifestations during treatment with TNF-alpha blockers: 11 cases. Ann Dermatol Venereol. 2007; 134:337–42.41. Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007; 56:69–83.
Article42. Callejas-Rubio JL, Ortego-Centeno N, Lopez-Perez L, Benticuaga MN. Treatment of therapy-resistant sarcoidosis with adalimumab. Clin Rheumatol. 2006; 25:596–7.
Article43. Utz JP, Limper AH, Kalra S, Specks U, Scott JP, Vuk-Pavlovic Z, et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003; 124:177–85.
Article44. Gonzá lez-López MA, Blanco R, González-Vela MC, Fernández-Llaca H, Rodríguez-Valverde V. Development of sarcoidosis during etanercept therapy. Arthritis Rheum. 2006; 55:817–20.
Article45. Peno-Green L, Lluberas G, Kingsley T, Brantley S. Lung injury linked to etanercept therapy. Chest. 2002; 122:1858–60.
Article46. Bremner R, Simpson E, White CR, Morrison L, Deodhar A. Palisaded neutrophilic and granulomatous dermatitis: an unusual cutaneous manifestation of immune-mediated disorders. Semin Arthritis Rheum. 2004; 34:610–6.
Article47. Deng A, Harvey V, Sina B, Strobel D, Badros A, Junkins-Hopkins JM, et al. Interstitial granulomatous dermatitis associated with the use of tumor necrosis factor alpha inhibitors. Arch Dermatol. 2006; 142:198–202.
Article48. Voulgari PV, Markatseli TE, Exarchou SA, Zioga A, Drosos AA. Granuloma annulare induced by anti-tumour necrosis factor therapy. Ann Rheum Dis. 2008; 67:567–70.
Article49. El Shabrawi-Caelen L, La Placa M, Vincenzi C, Haidn T, Muellegger R, Tosti A. Adalimumab-induced psoriasis of the scalp with diffuse alopecia: a severe potentially ir-reversible cutaneous side effect of TNF-alpha blockers. Inflamm Bowel Dis. 2010; 16:182–3.
Article50. Moran GW, Lim AW, Bailey JL, Dubeau MF, Leung Y, Devlin SM, et al. Review article: dermatological complications of immunosuppressive and anti-TNF therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2013; 38:1002–24.
Article51. Richardson SK, Gelfand JM. Immunobiologicals, cyto-kines, and growth factors in dermatology. Goldsmith LA, Fitzpatrick TB, editors. Fitzpatrick's dermatology in general medicine. 8th ed.p. 2819–22. New York: McGraw-Hill Medical;2012.52. Salliot C, Gossec L, Ruyssen-Witrand A, Luc M, Duclos M, Guignard S, et al. Infections during tumour necrosis factor-alpha blocker therapy for rheumatic diseases in daily practice: a systematic retrospective study of 709 patients. Rheumatology (Oxford). 2007; 46:327–34.53. Kim SY, Solomon DH. Tumor necrosis factor blockade and the risk of viral infection. Nat Rev Rheumatol. 2010; 6:165–74.
Article54. Sanz-Sánchez T, Daudén E, González-Arribas A, Garcí a-Díez A. Sudden onset of viral warts during treatment with etanercept. Actas Dermosifiliogr. 2010; 101:460–2.
Article55. Alpayci M. Exacerbation of verruca vulgaris associated with etanercept in a case of ankylosing spondylitis. Turk J Phys Med Rehab. 2013; 59:169.56. Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP. British Society for Rheumatology Biologics Register. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving antitumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006; 54:2368–76.
Article57. Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005; 52:3403–12.
Article58. Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002; 46:2287–93.
Article59. Mahé E, Descamps V, Grossin M, Fraitag S, Crickx B. CD30+ T-cell lymphoma in a patient with psoriasis treated with ciclosporin and infliximab. Br J Dermatol. 2003; 149:170–3.
Article60. Adams AE, Zwicker J, Curiel C, Kadin ME, Falchuk KR, Drews R, et al. Aggressive cutaneous T-cell lymphomas after TNFalpha blockade. J Am Acad Dermatol. 2004; 51:660–2.61. Sanli H, Ataman S, Akay BN, Yilmaz A, Yildizlar D, Gürgey E. Mycosis fungoides in a patient with ankylosing spondylitis during infliximab therapy. J Drugs Dermatol. 2007; 6:834–6.62. Wolfe F, Michaud K. The effect of methotrexate and antitumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007; 56:1433–9.
Article63. Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatolog Treat. 2004; 15:280–94.
Article64. Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002; 46:3151–8.
Article65. Klareskog L, Gaubitz M, Rodriguez-Valverde V, Malaise M, Dougados M, Wajdula J. Etanercept Study 301 Investigators. A longterm, open-label trial of the safety and efficacy of etanercept (Enbrel) in patients with rheumatoid arthritis not treated with other disease-modifying antirheumatic drugs. Ann Rheum Dis. 2006; 65:1578–84.
Article66. Lebwohl M, Blum R, Berkowitz E, Kim D, Zitnik R, Osteen C, et al. No evidence for increased risk of cutaneous squamous cell carcinoma in patients with rheumatoid arthritis receiving etanercept for up to 5 years. Arch Dermatol. 2005; 141:861–4.
Article67. Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999; 5:828–31.68. Penn I. Malignant melanoma in organ allograft recipients. Transplantation. 1996; 61:274–8.69. Jensen P, Hansen S, M⊘ller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin cancer in kidney and heart transplant recipients and different longterm immunosuppressive therapy regimens. J Am Acad Dermatol. 1999; 40:177–86.
Article70. Hollenbeak CS, Todd MM, Billingsley EM, Harper G, Dyer AM, Lengerich EJ. Increased incidence of melanoma in renal transplantation recipients. Cancer. 2005; 104:1962–7.
Article71. Le Mire L, Hollowood K, Gray D, Bordea C, Wojnarowska F. Melanomas in renal transplant recipients. Br J Dermatol. 2006; 154:472–7.
Article72. Fulchiero GJ Jr, Salvaggio H, Drabick JJ, Staveley-O'Carroll K, Billingsley EM, Marks JG, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007; 56(5 Suppl):S65–7.
Article73. Bovenschen HJ, Tjioe M, Vermaat H, de Hoop D, Witteman BM, Janssens RW, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006; 154:880–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-Tumor Necrosis Factor Monoclonal Antibody Treatment for Chronic Behcet's Disease: 3 Cases
- Role of Clusterin and Tumor Necrosis Factor Receptors on the Apoptosis of Prostate Cancer Cells
- Golimumab Therapy in Ulcerative Colitis
- A Case of Tumor Necrosis Factor-alpha Inhibitors-induced Pustular Psoriasis
- Preoperative use of anti-tumor necrosis factor therapy in Crohn's disease: promises and pitfalls