J Rheum Dis.
2016 Feb;23(1):30-36. 10.4078/jrd.2016.23.1.30.
Two-year Follow-up Study of the Relationship between the Changes of Serum Homocysteine and Those of Serum Uric Acid Levels, Lipid Profiles and Renal Function in Gout Patients
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea. beconst@cau.ac.kr
- KMID: 2222798
- DOI: http://doi.org/10.4078/jrd.2016.23.1.30
Abstract
OBJECTIVE
Gout is known to be associated with cardiovascular disease (CVD), and hyperhomocysteinemia is one of the risk factors for CVD. We investigated the associations between the change of serum homocysteine (Hcy) level and those of the other parameters including serum uric acid level, renal function, and cholesterol profiles in chronic gout patients with longitudinal follow-up data.
METHODS
Ninety-one male patients with chronic gout and 97 age-matched healthy male control subjects were included in the previous study. Among them, 33 patients with gout and 39 healthy control subjects underwent follow-up tests for Hcy levels with an average of 24.00+/-9.12 months in this study.
RESULTS
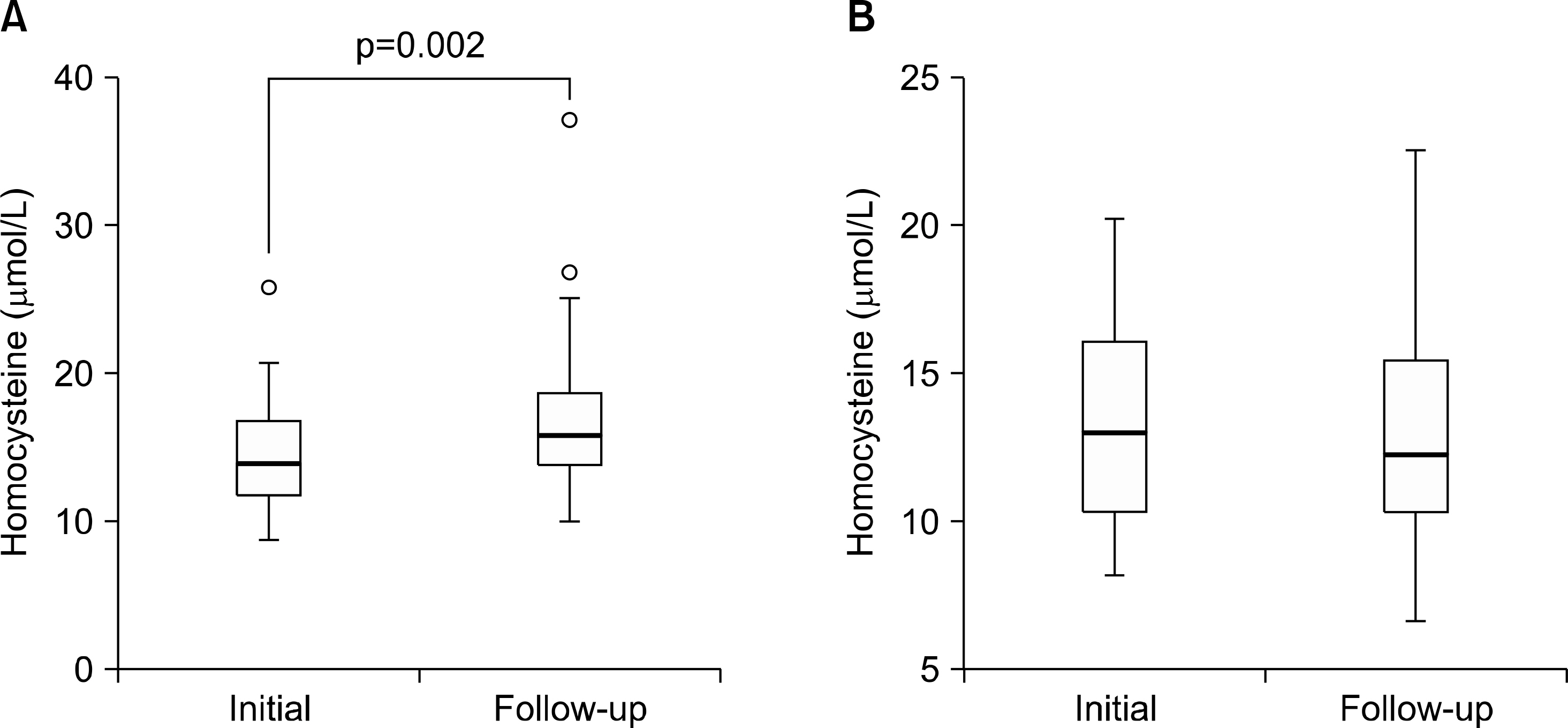
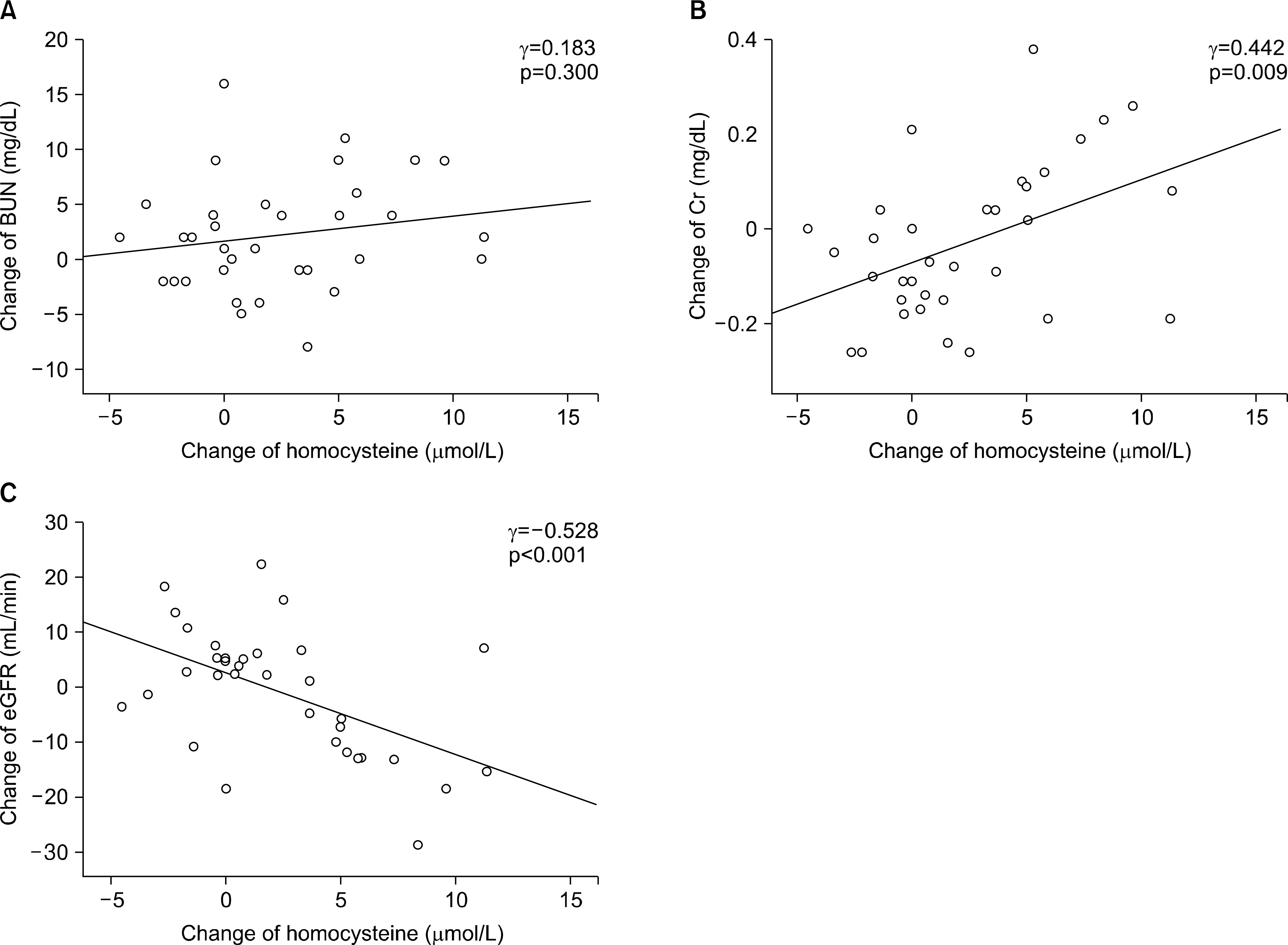
The follow-up data showed that gout patients had significantly higher levels of Hcy in serum than control subjects (16.75+/-5.43 micromol/L vs. 13.17+/-3.83 micromol/L, p=0.002). In gout patients, the change of serum Hcy level after follow up showed positive correlation with the change of creatinine (gamma=0.442, p=0.009) and negative correlation with estimated glomerular filtration rate (eGFR; gamma=-0.528, p<0.001). However, the change of serum Hcy level did not show correlation with the changes of uric acid level or the lipid profiles.
CONCLUSION
Serum Hcy level was elevated in gout patients compared with control subjects. The change of serum Hcy level showed negative correlation with the change of eGFR. Hyperhomocysteinemia in gout patients was associated with decreased renal function, but not with serum uric acid or lipid profiles.
MeSH Terms
Figure
Reference
-
1. Puig JG, Martínez MA. Hyperuricemia, gout and the metabolic syndrome. Curr Opin Rheumatol. 2008; 20:187–91.
Article2. Kuo CF, See LC, Luo SF, Ko YS, Lin YS, Hwang JS, et al. Gout: an independent risk factor for all-cause and cardiovascular mortality. Rheumatology (Oxford). 2010; 49:141–6.
Article3. Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005; 67:1739–42.
Article4. Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000; 321:199–204.
Article5. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995; 10:111–3.
Article6. Kang SS. Critical points for determining moderate hyper-homocyst(e)inaemia. Eur J Clin Invest. 1995; 25:806–8.
Article7. van Guldener C, Stam F, Stehouwer CD. Hyperhomocys-teinaemia in chronic kidney disease: focus on transme-thylation. Clin Chem Lab Med. 2005; 43:1026–31.
Article8. Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003; 138:891–7.
Article9. Majors A, Ehrhart LA, Pezacka EH. Homocysteine as a risk factor for vascular disease. Enhanced collagen production and accumulation by smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997; 17:2074–81.10. Slot O. Homocysteine, a marker of cardiovascular disease risk, is markedly elevated in patients with gout. Ann Rheum Dis. 2013; 72:457.11. Cheng TT, Lai HM, Chang HW, Luo SF. Elevated serum homocysteine levels for gouty patients. Clin Rheumatol. 2005; 24:103–6.
Article12. Choi ST, Kim JS, Song JS. Elevated serum homocysteine levels were not correlated with serum uric acid levels, but with decreased renal function in gouty patients. J Korean Med Sci. 2014; 29:788–92.
Article13. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977; 20:895–900.
Article14. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–70.15. Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham study. J Clin Epidemiol. 1988; 41:237–42.
Article16. Chen SY, Chen CL, Shen ML. Severity of gouty arthritis is associated with Q-wave myocardial infarction: a large-scale, cross-sectional study. Clin Rheumatol. 2007; 26:308–13.
Article17. Full LE, Ruisanchez C, Monaco C. The inextricable link between atherosclerosis and prototypical inflammatory diseases rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2009; 11:217.
Article18. Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. MRFIT Research Group. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008; 168:1104–10.
Article19. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007; 116:894–900.
Article20. Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary artery risk development in young adults. Ann Epidemiol. 1998; 8:250–61.21. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the third National Health and Nutrition examination survey. Arthritis Rheum. 2007; 57:109–15.
Article22. Rho YH, Choi SJ, Lee YH, Ji JD, Choi KM, Baik SH, et al. The prevalence of metabolic syndrome in patients with gout: a multicenter study. J Korean Med Sci. 2005; 20:1029–33.
Article23. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008; 359:1811–21.
Article24. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007; 293:C584–96.
Article25. Bhakdi S, Torzewski M, Klouche M, Hemmes M. Complement and atherogenesis: binding of CRP to degraded, non-oxidized LDL enhances complement activation. Arterioscler Thromb Vasc Biol. 1999; 19:2348–54.26. Hayden MR, Tyagi SC. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutr Metab (Lond). 2004; 1:10.27. Kelly PJ, Rosand J, Kistler JP, Shih VE, Silveira S, Plomaritoglou A, et al. Homocysteine, MTHFR 677C→ T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology. 2002; 59:529–36.28. Iso H, Moriyama Y, Sato S, Kitamura A, Tanigawa T, Yamagishi K, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004; 109:2766–72.
Article29. McCully KS. Homocysteine and vascular disease. Nat Med. 1996; 2:386–9.
Article30. Hayashi T, Honda G, Suzuki K. An atherogenic stimulus homocysteine inhibits cofactor activity of thrombomodulin and enhances thrombomodulin expression in human umbilical vein endothelial cells. Blood. 1992; 79:2930–6.
Article31. Hajjar KA. Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. J Clin Invest. 1993; 91:2873–9.
Article32. Lentz SR, Sadler JE. Inhibition of thrombomodulin surface expression and protein C activation by the thrombogenic agent homocysteine. J Clin Invest. 1991; 88:1906–14.
Article33. Craswell PW, Price J, Boyle PD, Heazlewood VJ, Baddeley H, Lloyd HM, et al. Chronic renal failure with gout: a marker of chronic lead poisoning. Kidney Int. 1984; 26:319–23.
Article34. Batuman V, Maesaka JK, Haddad B, Tepper E, Landy E, Wedeen RP. The role of lead in gout nephropathy. N Engl J Med. 1981; 304:520–3.
Article35. Avram Z, Krishnan E. Hyperuricaemia− where nephrology meets rheumatology. Rheumatology (Oxford). 2008; 47:960–4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Elevated Serum Homocysteine Levels Were Not Correlated with Serum Uric Acid Levels, but with Decreased Renal Function in Gouty Patients
- Study on Effect of Benzbromarone in the Patients with Gout
- The Relationship between Homocysteine and Uric Acid Levels in Gouty Patients
- Pharmacotherapy for gout
- Management of Complicated Gout