J Korean Ophthalmol Soc.
2012 Aug;53(8):1068-1075. 10.3341/jkos.2012.53.8.1068.
Clinical Efficacy of Topical Voriconazole as Treatment in Culture-Positive Fungal Keratitis
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea. kmk9@snu.ac.kr
- 2Laboratory of Corneal Regenerative Medicine and Ocular Immunology, Seoul Artificial Eye Center, Seoul National University Hospital Biomedical Research Institute, Seoul, Korea.
- KMID: 2215947
- DOI: http://doi.org/10.3341/jkos.2012.53.8.1068
Abstract
- PURPOSE
To evaluate the efficacy of topical voriconazole in treatment of culture-positive fungal keratitis.
METHODS
Twenty-two cases of culture-positive fungal keratitis treated with topical anti-fungal drugs from October 2004 to December 2010 were evaluated and medical records were reviewed. Patients were divided into 3 groups according to the method of topical antifungal treatment; the first group (V, n = 6) received voriconazole monotherapy, the second group (V+, n = 10) received topical voriconazole and amphotericin B combined therapy, and the third group (A, n = 6) received amphotericin B monotherapy.
RESULTS
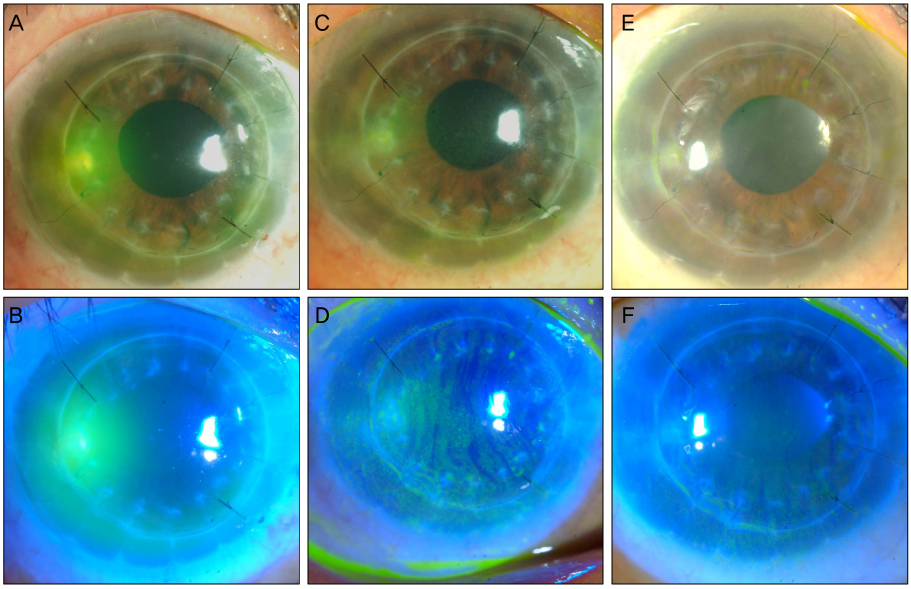
The treatment success rate between the 3 groups was not significantly different. The epithelial defect healing time of group V was shorter than group V+. Treatment failure risk factors included Fusarium spp. infection and the presence of hypopyon. Eight eyes received topical voriconazole maintenance therapy for 66 days and there were no corneal erosions or epithelial defects.
CONCLUSIONS
In fungal keratitis, topical voriconazole is as effective as other standard treatments and provides a faster healing time in the corneal epithelium than other standard treatments. However, voriconazole should be used with caution and therapeutic surgery should be considered in Fusarium spp. infections.
Keyword
MeSH Terms
Figure
Reference
-
1. Kernt M, Kampik A. Intracameral voriconazole: in vitro safety for human ocular cells. Toxicology. 2009. 258:84–93.2. Hariprasad SM, Mieler WF, Lin TK, et al. Voriconazole in the treatment of fungal eye infections: a review of current literature. Br J Ophthalmol. 2008. 92:871–878.3. Marangon FB, Miller D, Giaconi JA, Alfonso EC. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004. 137:820–825.4. Hariprasad SM, Mieler WF, Holz ER, et al. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol. 2004. 122:42–47.5. Lau D, Fedinands M, Leung L, et al. Penetration of voriconazole, 1%, eyedrops into human aqueous humor: a prospective open-label study. Arch Ophthalmol. 2008. 126:343–346.6. Vemulakonda GA, Hariprasad SM, Mieler WF, et al. Aqueous and vitreous concentrations following topical administration of 1% voriconazole in humans. Arch Ophthalmol. 2008. 126:18–22.7. Kim KH, Kim MJ, Tchah H. Management of fungal ocular infection with topical and intracameral voriconazole. J Korean Ophthalmol Soc. 2008. 49:1054–1060.8. Lee SJ, Lee JJ, Kim SD. Topical and oral voriconazole in the treatment of fungal keratitis. Korean J Ophthalmol. 2009. 23:46–48.9. Seo JH, Wee WR, Lee JH, Kim MK. Risk factors affecting efficacy of intracameral amphotericin injection in deep keratomycosis. J Korean Ophthalmol Soc. 2007. 48:1201–1211.10. Baldinger J, Doft BH, Burns SA, Johnson B. Retinal toxicity of amphotericin B in vitrectomised versus non-vitrectomised eyes. Br J Ophthalmol. 1986. 70:657–661.11. Lin CP, Boehnke M. Effect of fortified antibiotic solutions on corneal epithelial wound healing. Cornea. 2000. 19:204–206.12. O'Day DM, Head WS, Robinson RD, Clanton JA. Corneal penetration of topical amphotericin B and natamycin. Curr Eye Res. 1986. 5:877–882.13. Al-Badriyeh D, Neoh CF, Stewart K, Kong DC. Clinical utility of voriconazole eye drops in ophthalmic fungal keratitis. Clin Ophthalmol. 2010. 4:391–405.14. Han SB, Shin YJ, Hyon JY, Wee WR. Cytotoxicity of voriconazole on cultured human corneal endothelial cells. Antimicrob Agents Chemother. 2011. 55:4519–4523.15. Chang CH, Lin CP, Wang HZ. Cytotoxicity of intracameral injection drugs to corneal endothelium as evaluated by corneal endothelial cell culture. Cornea. 1995. 14:71–76.16. Dupuis A, Tournier N, Le Moal G, Venisse N. Preparation and stability of voriconazole eye drop solution. Antimicrob Agents Chemother. 2009. 53:798–799.17. Al-Badriyeh D, Li J, Stewart K, et al. Stability of extemporaneously prepared voriconazole ophthalmic solution. Am J Health Syst Pharm. 2009. 66:1478–1483.18. Gupta S, Shrivastava RM, Tandon R, et al. Role of voriconazole in combined acanthamoeba and fungal corneal ulcer. Cont Lens Anterior Eye. 2011. 34:287–289.19. Shen YC, Wang CY, Tsai HY, Lee HN. Intracameral voriconazole injection in the treatment of fungal endophthalmitis resulting from keratitis. Am J Ophthalmol. 2010. 149:916–921.20. Szabo Z, Borbely A, Kardos G, et al. In vitro efficacy of amphotericin B, 5-fluorocytosine, fluconazole, voriconazole and posaconazole against Candida dubliniensis isolates using time-k2ill methodology. Mycoses. 2010. 53:196–199.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Fungal Ocular Infection with Topical and Intracameral Voriconazole
- Topical and Oral Voriconazole in the Treatment of Fungal Keratitis
- Clinical Effects of Intracameral Voriconazole Injection in Patients with Fungal Keratitis Refractory to Conventional Treatment
- A case of candida albicans-induced fungal keratitis in a Chihuahua dog – with a focus on optical coherence tomographic features
- A Case of Fungal Keratitis Scedosporium apiospermum