J Korean Ophthalmol Soc.
2015 Nov;56(11):1677-1683. 10.3341/jkos.2015.56.11.1677.
Comparison of the Effectiveness between Sampling Methods for Protein Analysis of Tear Fluids
- Affiliations
-
- 1The Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. shadik@yuhs.ac
- 2Corneal Dystrophy Research Institute, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2214174
- DOI: http://doi.org/10.3341/jkos.2015.56.11.1677
Abstract
- PURPOSE
Although various sampling methods of tears from conjunctival sac have been reported, no previous study compared their effectiveness or efficiency based on protein extraction. By comparing the compliance, volume and protein concentration of each tear sampling method, we searched for the most efficient tear collection method.
METHODS
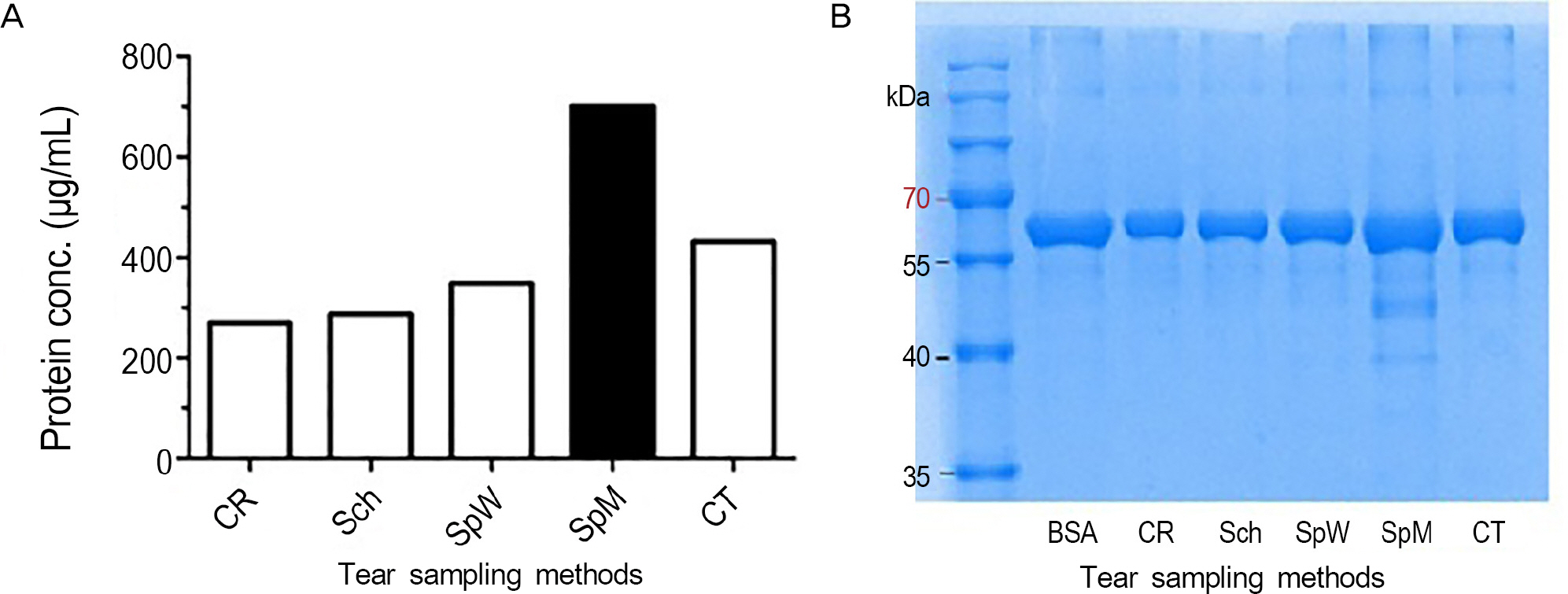
Resting tear samples of 14 eyes of normal subjects were collected using Schirmer paper, capillary tube, cellolose acetate rod and 3 different ophthalmic sponges made of different materials and density (Merocel(R), KeraCel(R) and Weck-Cel(R)). After centrifugation of the collected tear samples, the tear volume and protein concentration were measured for each method. Additionally, the compliance of each tear sampling method was analyzed by numerically representing the amount of discomfort experienced during resting tear collection.
RESULTS
The average volume retrieved by each tear sampling method was 9.0 +/- 1.1 microL with no significant differences between groups. The average concentration of protein retrieved by each tear sampling method was 5.3 +/- 1.2 microg/microL. Merocel(R) retrieved 7.6 +/- 0.61 microg/microL, which was significantly higher than other sampling methods (p < 0.05). The compliance of Merocel(R) and the capillary tube were the highest, while KeraCel(R) showed the lowest compliance.
CONCLUSIONS
Merocel(R) retrieved the highest amount of protein and showed high compliance and may be the most effective and easily applicable tear sampling method in clinical settings.
Figure
Reference
-
References
1. Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clin Chim Acta. 2006; 369:17–28.
Article2. Wu K, Zhang Y. Clinical application of tear proteomics: present and future prospects. Proteomics Clin Appl. 2007; 1:972–82.
Article3. Lam SM, Tong L, Duan X. . Extensive characterization of hu-man tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. 2014; 55:289–98.
Article4. de Souza GA, Godoy LM, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006; 7:R72.5. Soria J, Durán JA, Etxebarria J. . Tear proteome and protein network analyses reveal a novel pentamarker panel for tear film characterization in dry eye and meibomian gland dysfunction. J Proteomics. 2013; 78:94–112.
Article6. Li B, Sheng M, Li J. . Tear proteomic analysis of Sjögren syn-drome patients with dry eye syndrome by two-dimensional- nano-liquid chromatography coupled with tandem mass spectrometry. Sci Rep. 2014; 4:5772.
Article7. Versura P, Nanni P, Bavelloni A. . Tear proteomics in evapo-rative dry eye disease. Eye (Lond). 2010; 24:1396–402.
Article8. Chae JK, Park SP, Choi TH. Comparative analysis of the tear pro-tein expression after photorefractive keratectomy using two-di-mensional electrophoresis. J Korean Ophthalmol Soc. 2009; 50:762–8.
Article9. Zhou L, Beuerman RW, Chan CM. . Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009; 8:4889–905.
Article10. Labbé A, Brignole-Baudouin F, Baudouin C. Ocular surface inves-tigations in dry eye. J Fr Ophtalmol. 2007; 30:76–97.11. Quah JH, Tong L, Barbier S. Patient acceptability of tear collection in the primary healthcare setting. Optom Vis Sci. 2014; 91:452–8.
Article12. Posa A, Bräuer L, Schicht M. . Schirmer strip vs. capillary tube method: non-invasive methods of obtaining proteins from tear fluid. Ann Anat. 2013; 195:137–42.
Article13. Esmaeelpour M, Cai J, Watts P. . Tear sample collection using cellulose acetate absorbent filters. Ophthalmic Physiol Opt. 2008; 28:577–83.
Article14. Inic-Kanada A, Nussbaumer A, Montanaro J. . Comparison of ophthalmic sponges and extraction buffers for quantifying cyto-kine profiles in tears using Luminex technology. Mol Vis. 2012; 18:2717–25.15. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92.16. Stuchell RN, Feldman JJ, Farris RL, Mandel ID. The effect of col-lection technique on tear composition. Invest Ophthalmol Vis Sci. 1984; 25:374–7.17. Gachon AM, Richard J, Dastugue B. Human tears: normal protein pattern and individual protein determinations in adults. Curr Eye Res 1982-1983. 2:301–8.
Article18. Ng V, Cho P, To C. Tear proteins of normal young Hong Kong Chinese. Graefes Arch Clin Exp Ophthalmol. 2000; 238:738–45.
Article19. López-Cisternas J, Castillo-Díaz J, Traipe-Castro L, López-Solís RO. Use of polyurethane minisponges to collect human tear fluid. Cornea. 2006; 25:312–8.
Article20. Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Prog Retin Eye Res. 2012; 31:527–50.
Article21. Hyon SH, Cha WI, Ikada Y. . Poly(vinyl alcohol) hydrogels as soft contact lens material. J Biomater Sci Polym Ed. 1994; 5:397–406.
Article22. Alves MH, Jensen BE, Smith AA, Zelikin AN. Poly(vinyl alcohol) physical hydrogels: new vista on a long serving biomaterial. Macromol Biosci. 2011; 11:1293–313.
Article23. Bao CY, Long DR, Vergelati C. Miscibility and dynamical proper-ties of cellulose acetate/plasticizer systems. Carbohydr Polym. 2015; 116:95–102.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Tear Composition in Chronic Blepharitis
- The Quantitative Analysis of Tear Total Protein and Lysozyme in Normal and Diabetes
- Immunoelectrophoretic Analysis of Blister Fluids
- Comparison of Red Blood Cell, White Blood Cell and Differential Counts between UF-5000 System and Manual Method
- Chromosome Analysis of Ascitic Fluids from Patients with Malignant Tumor