J Korean Ophthalmol Soc.
2009 Jul;50(7):1027-1034. 10.3341/jkos.2009.50.7.1027.
Short-term Efficacy of Intravitreal Ranibizumab for Myopic Choroidal Neovascularization
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, College of Medicine, The Catholic University of Korea, Seoul, Korea. youngjungroh@hanmail.net
- KMID: 2212490
- DOI: http://doi.org/10.3341/jkos.2009.50.7.1027
Abstract
- PURPOSE
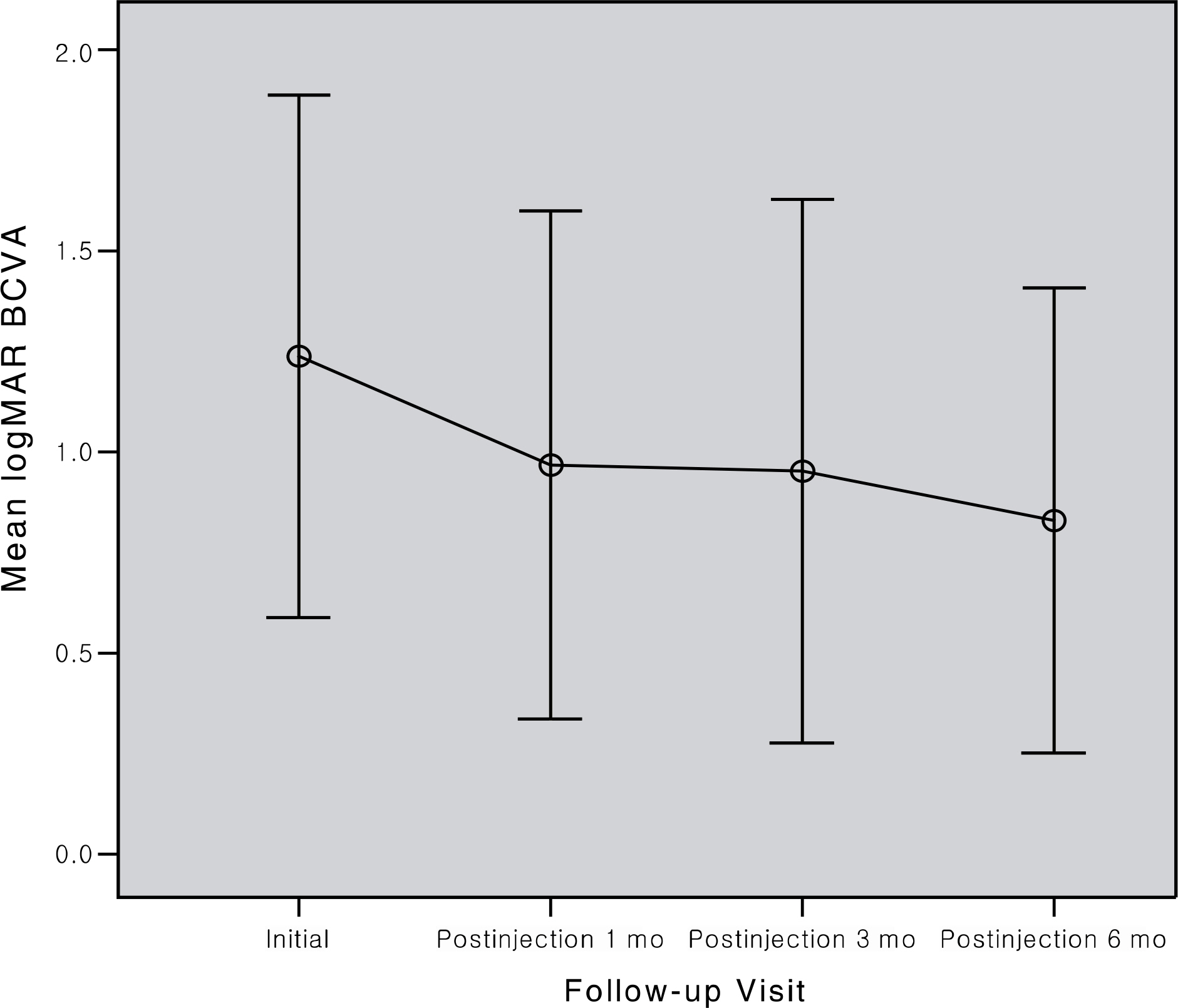
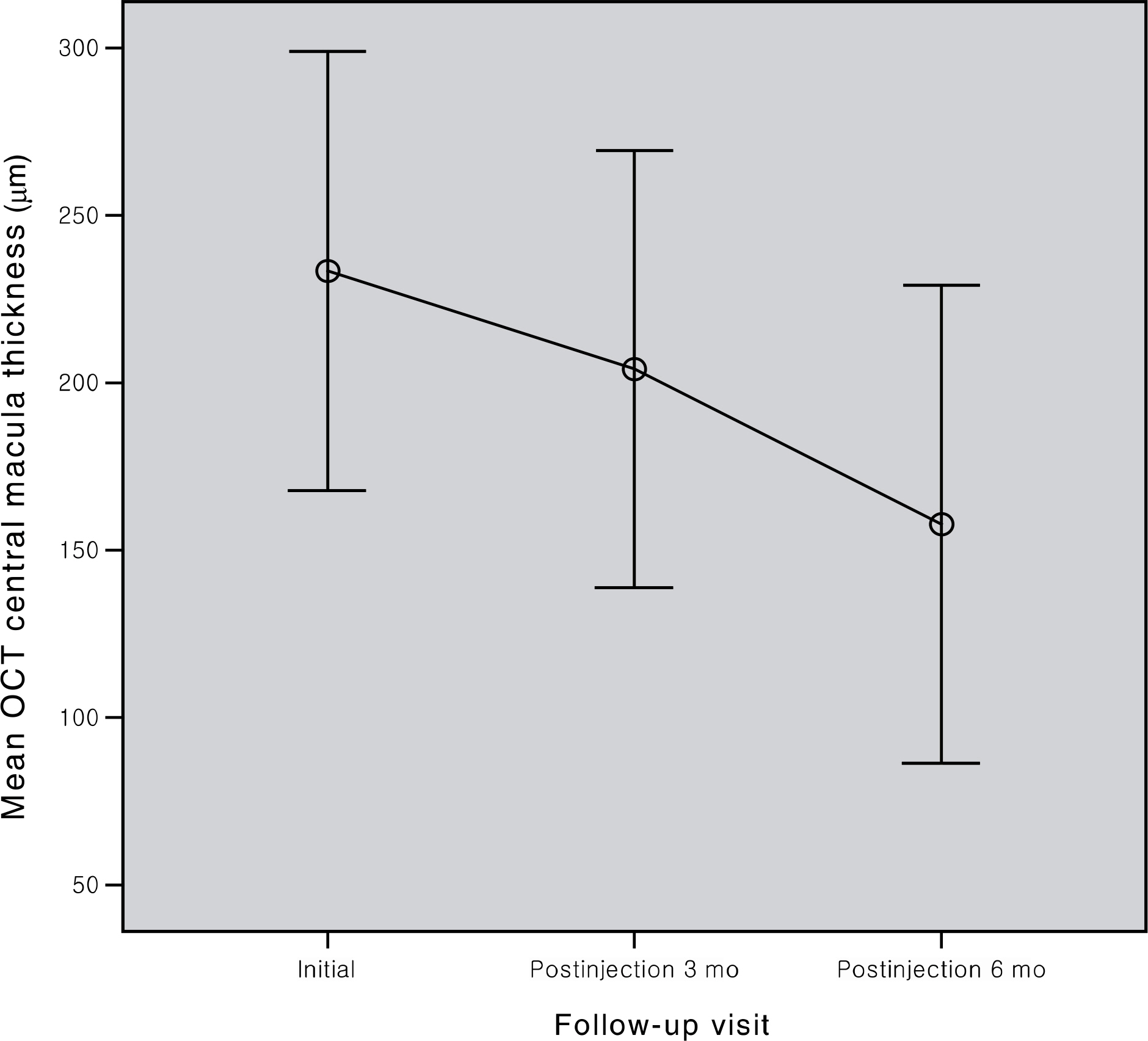
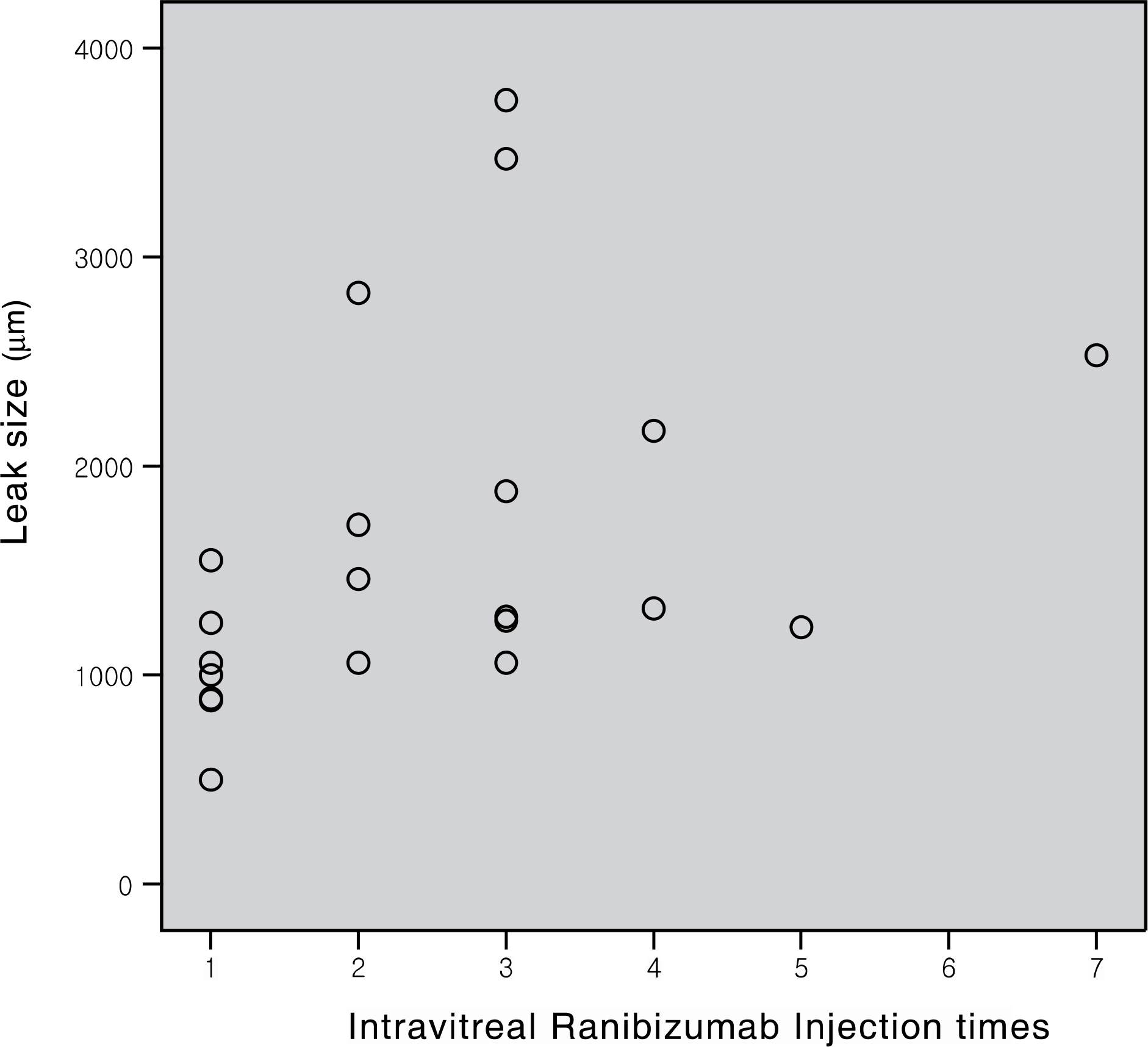
To evaluate the effects of intravitreal ranibizumab in myopic choroidal neovascularization (CNV). METHODS: Patients who underwent intravitreal ranibizumab injection for myopic CNV, and were followed up more than 6 months, and their records were retrospectively investigated. The best corrected visual acuity, central macular thickness, and leak in fluorescein angiography were compared at baseline, and at 1, 3, and 6 months after injection. RESULTS: Twenty-one eyes of 18 patients were evaluated. The mean best corrected visual acuity (logMAR) was 1.23+/-0.65, 0.96+/- 0.40, 0.95+/-0.67, and 0.83+/-0.58 at baseline, 1, 3, and 6 months, respectively (p<0.001, p=0.006, p=0.001). The mean central macular thickness was 233.42+/-65.55 microm, 204.14+/-65.29 micrometer, and 157.76+/-71.45 microm at baseline, 3, and 6 months, respectively (p<0.001). In fluorescein angiography at 6 months after injection, regression was observed in 12 eyes, and fibrosis in 9 eyes. CONCLUSIONS: Intravitreal ranibizumab injection for myopic CNV in Korean patients appeared to be effective, resulting in regression of lesion and improvement of visual acuity.
MeSH Terms
Figure
Cited by 1 articles
-
Risk Factors for Retinal Pigment Epithelium Tears after Anti-VEGF Agent Injection in Age-Related Macular Degeneration
Woo Seok Choae, Jae Hong Park, Woo Seok Lee, Sang Won Kim, Hee Seong Yoon
J Korean Ophthalmol Soc. 2013;54(10):1546-1553. doi: 10.3341/jkos.2013.54.10.1546.
Reference
-
References
1. Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992; 12:127–33.
Article2. Hotchkiss ML, Green WR. Pathologic myopia and choroidal neovascularization. Am J Ophthalmol. 1981; 91:177–83.
Article3. Tabandeh H, Flynn HW Jr, Scott IU, et al. Visual acuity outcomes of patients 50 years of age and older with high myopia and untreated choroidal neovascularization. Ophthalmology. 1999; 106:2063–7.
Article4. Yoshida T, Ohno-Matsui K, Ohtake Y, et al. Long-term visual prognosis of choroidal neovascularization in high myopia: a comparison between age groups. Ophthalmology. 2002; 109:712–9.5. Ryu IH, Kim BG, Lee SC. Photodynamic therapy of subfoveal choroidal neovascualrization in pathologic myopia. J Korean Ophthalmol Soc. 2003; 44:1991–5.6. Verteporfin in Photodynamic Therapy Study Group. Photo-dynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 1-year results of a randomized clinical trial—VIP report no. 1. Ophthalmology. 2001; 108:841–52.7. Blinder KJ, Blumenkranz MS, Bressler NM, et al. Verteporfin therapy of subfoveal choroidal neovascularisation in pathologic myopia: 2-year results of a randomized clinical trial—VIP report no. 3. Ophthalmology. 2003; 110:667–73.8. Emerson MV, Lauer AK, Flaxel CJ, et al. Intravitreal bevacizumab (Avastin) treatment of neovascular age-related macular degeneration. Retina. 2007; 27:439–44.
Article9. Goff MJ, Johnson RN, McDonald HR, et al. Intravitreal bevacizumab for previously treated choroidal neovascularization from age-related macular degeneration. Retina. 2007; 27:432–8.
Article10. Lazic R, Gabric N. Intravitreally administered bevacizumab (Avastin) in minimally classic and occult choroidal neovascularization secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007; 245:68–73.
Article11. Costa RA, Jorge R, Calucci D, et al. Intravitreal Bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006; 47:4569–78.
Article12. Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular agerelated macular degeneration. Retina. 2006; 26:495–511.
Article13. Hernandez-Rojas ML, Quiroz-Mercado H, Dalma-Weiszhausz J, et al. Short-term effects of intravitreal bevacizumab for subfoveal choroidal neovascularization in pathologic myopia. Retina. 2007; 27:707–12.14. Ruiz-Moreno JM, Gomez-Ulla F, Montero JA, et al. Intravitreous bevacizumab to treat subfoveal choroidal neovascularization in highly myopic eyes: short-term results. Eye. 2009; 23:334–8.
Article15. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.
Article16. Silva RM, Ruiz-Moreno JM, Nascimento J, et al. Short-term efficacy and safety of intravitreal Ranibizumab for myopic choroidal neovascularization. Retina. 2008; 28:1117–23.
Article17. Brancato R, Pece A, Avanza P, Radrizzani E. Photocoagulation scar expansion after laser therapy for choroidal neovascularization in degenerative myopia. Retina. 1990; 10:239–43.
Article18. Ladas ID, Moschos MM, Rouvas AA, et al. Lacquer crack formation after photodynamic therapy. Eur J Ophthalmol. 2003; 13:729–33.
Article19. Ohno-Matsui K, Moriyama M, Hayashi K, Mochizuki M. Choroidal vein and artery occlusion following photodynamic therapy in eyes with pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 2006; 244:1363–6.
Article20. Yamamoto I, Rogers AH, Reichel E, et al. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathological myopia. Br J Ophthalmol. 2007; 91:157–60.
Article21. Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB. Intravitreal Bevacizumab Treatment for Choroidal Neovascularization in Pathologic Myopia: 12-month Results. Am J Ophthalmol. 2009; 147:84–93.
Article22. Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: six-month results of a prospective pilot study. Ophthalmology. 2007; 114:2190–6.23. Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal Bevacizumab for Choroidal Neovascularization Attributable to Pathological Myopia: One-Year Results. Am J Ophthalmol. 2009; 147:94–100.
Article24. Arias L, Planas N, Prades S, et al. Intravitreal bevacizuamb for choroidal neovascularisation secondary to pathological myopia: 6-month results. Br J Ophthalmol. 2008; 92:1035–9.25. Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: phase III clinical trial results. Ophthalmol Clin North Am. 2006; 19:361–72.26. Kojima A, Ohno-Matsui K, Teramukai S, et al. Estimation of visual outcome without treatment in patients with subfoveal choroidal neovascularization in pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 2006; 244:1474–9.
Article27. Axer-Siegel R, Ehrlich R, Weinberger D, et al. Photodynamic therapy of subfoveal choroidal neovascularization in high myopia in a clinical setting: visual outcome in relation to age. Am J Ophthalmol. 2004; 138:602–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Therapeutic Effect of Intravitreal Bevacizumab (Avastin) on Myopic Choroidal Neovascularization
- Recurrent Idiopathic Choroidal Neovascularization in an Adolescent
- Long-Term Effect of Intravitreal Ranibizumab Injection on Choroidal Neovascularization in Age-Related Macular Degeneration
- Macular Hole Following Intravitreal Ranibizumab Injections for Choroidal Neovascularization
- Effect of High-dose Intravitreal Bevacizumab Injection on Refractory Idiopathic Choroidal Neovasculariz