J Korean Ophthalmol Soc.
2009 Feb;50(2):303-307. 10.3341/jkos.2009.50.2.303.
Confocal Microscopic Changes in the Cornea 10 Years After Photorefractive Keratectomy
- Affiliations
-
- 1Department of Ophthalmology, School of Medicine, Pusan National University, Pusan, Korea. jongsool@pusan.ac.kr
- KMID: 2212068
- DOI: http://doi.org/10.3341/jkos.2009.50.2.303
Abstract
-
PURPOSE: The present study compares, using a new generation high-resolution in vivo confocal microscope, the corneas of patients who underwent photorefractive keratectomy (PRK) 10 years previously with those of healthy persons.
CASE SUMMARY
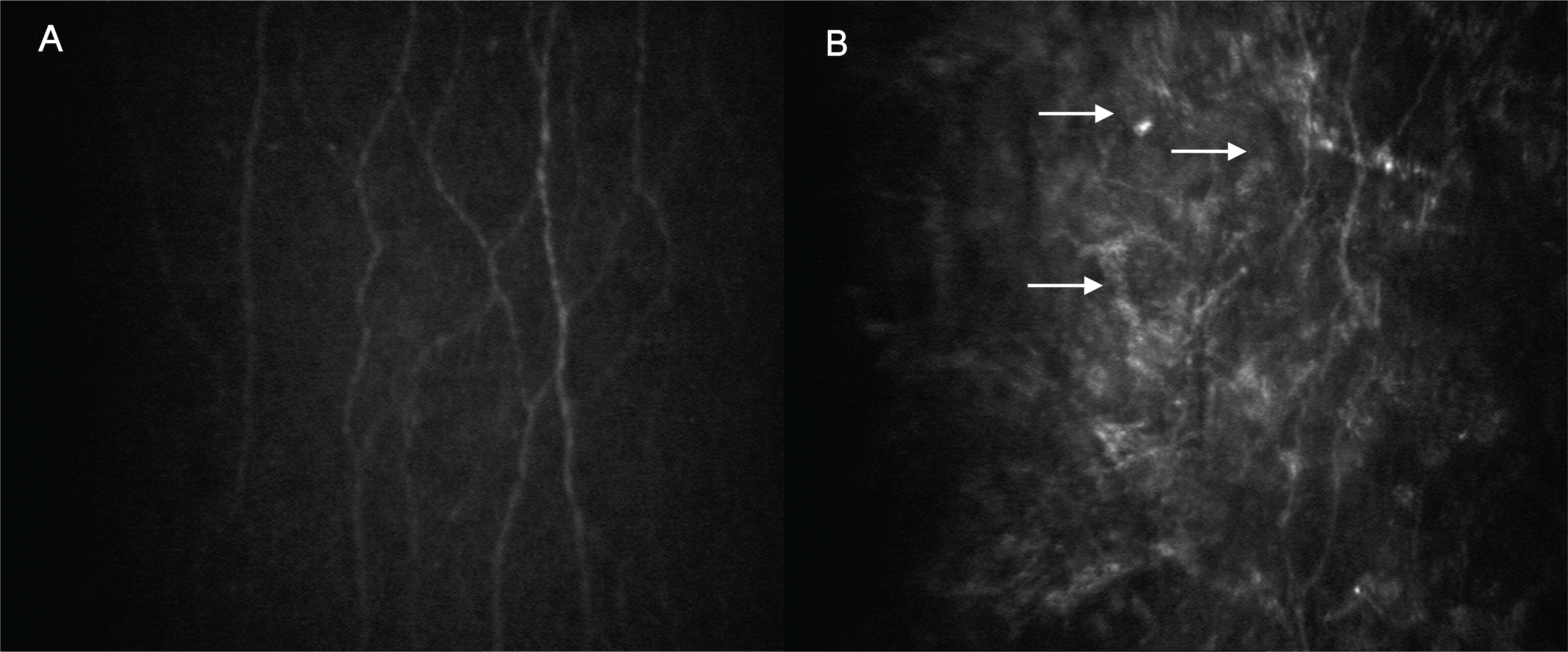
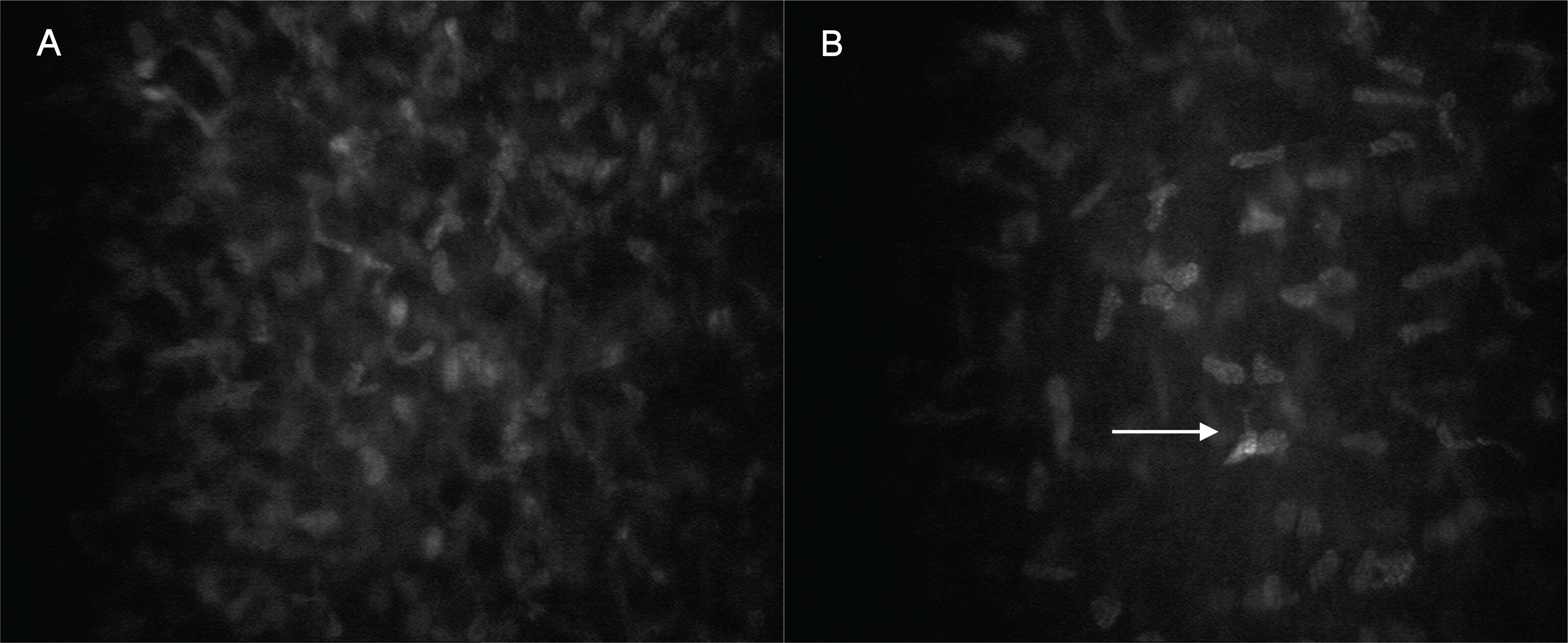
A confocal microscope (Confoscan 4.0, Fortune Technology, Italy) was used to get the data from healthy volunteers and patients. Corneal cross-sectional images of the epithelium, Bowman's layer, stromal layer (anterior, middle and posterior keratocyte), Descemet's membrane, and endothelium were compared. In PRK corneas, the superficial epithelium was nearly intact and the subbasal nerve plexus was visible, but some hyperreflective areas were also found in the nerve plexus. Because of the absence of the Bowman's layer, some ECM and keratocytes were visualized in their optical section. Although anterior keratocytes showed uneven distribution with less cellularity, middle and posterior keratocytes looked unaffected. Likewise, there were no differences in the endothelium between the two groups.
CONCLUSIONS
Ten years after PRK, the subbasal nerve plexus and anterior keratocytes showed histologic changes after corneal wound recovery.
MeSH Terms
Figure
Reference
-
References
1. Trokel SL, Srinnivasan R, Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. 1983; 96:710–5.
Article2. Epstein D, Fagerholm P, Hamberg-Nyström H, Tengroth B. Twenty-four month follow-up of excimer laser photorefractive keratectomy for myopia: refractive and visual acuity results. Ophthalmology. 1994; 101:1558–63.3. Dutt S, Steinert RF, Raizman MB, Puliafito CA. One-year results of excimer laser photorefractive keratectomy for low to moderate myopia. Arch Ophthalmol. 1994; 112:1427–36.
Article4. Kim JH, Kim MS, Hahn TW, et al. Five year results of photo-refractive keratectomy for myopia. J Cataract Refract Surg. 1997; 23:731–5.
Article5. Stephenson CG, Gartry DS, O'Brart DP, et al. Photorefractive keratectomy: a 6-year follow-up study. Ophthalmology. 1998; 105:273–81.6. Moilanen JA, Vesaluoma MH, Müller LJ, Tervo TM. Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003; 44:1064–9.
Article7. Erie JC, McLaren JW, Hodge DO, Bourne WM. Long-term corneal keratoctye deficits after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2005; 103:56–68.8. Rosenberg ME, Tervo TM, Petroll WM, Vesaluoma MH. In vivo confocal microscopy of patient with corneal recurrent erosion syndrome or epithelial basement membrane dystrophy. Ophthalmology. 2000; 107:565–73.9. Rosenberg ME, Tervo TM, Immonen IJ, et al. Corneal structure and sensitivity in typeⅠ diabetes mellitus. Invest Ophthalmol Vis Sci. 2000; 41:2915–21.10. Linna T, Tervo T. Real-time confocal microscopic observations on human corneal nerves and wound healing after excimer laser photorefractive keratectomy. Curr Eye Res. 1997; 16:640–49.
Article11. Frueh BE, Cadez R, Böhnke M. In vivo confocal microscopy after photorefractive keratectomy in humans: a prospective, long-term study. Arch Ophthalmol. 1998; 116:1425–31.12. Erie JC. Corneal wound healing after photorefractive keratectomy: a 3-year confocal microscopy study. Trans Am Ophthalmol Soc. 2003; 101:287–328.13. Ozdamar A, Aras C, Karakas N, et al. Changes in tear flow and tear film stability after photorefractive keratectomy. Cornea. 1999; 18:437–9.
Article14. Seiler T, McDonnell P. Excimer laser photorefractive keratectomy. Surv Ophthalmol. 1995; 40:89–118.
Article15. Li DQ, Tseng SC. Three patterns of cytokine expression potentially invlolved in epithelial-fibroblast interactions of human ocular surface. J Cell Phys. 1995; 163:61–79.16. Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000; 107:1235–45.
Article17. Lohmann C, Gartry D, Kerr Muir M, et al. “Haze” in photorefractive keratectomy; its origins and consequences. Laser Light Ophthalmol. 1991; 4:15–34.18. Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999; 18:293–309.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corneal wound Healing Following Excimer Laser Photorefractive Keratectomy Using Pressure Patch, T-lens and Collagen Shield on Rabbit Cornea
- Complications after Excimer Laser Photorefractive Keratectomy in Myopia
- Topographic Changes of Posterior Corneal Surface after Photorefractive Keratectomy with Orbscan II(R) Topography
- The Comparison of the Effect of Therapeutic Lrens, Collagen Shield, and Tarsorrhaphy on Stromal Inflammation after Photorefractive Keratectomy in a Rabbit model
- A Case of Ocular Deviation after Excinier Laser Photorefractive Keratectomy