J Korean Ophthalmol Soc.
2009 Feb;50(2):285-289. 10.3341/jkos.2009.50.2.285.
Distribution of Myosin Heavy Chain Isoform in Human Extraocular Muscles
- Affiliations
-
- 1Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. syoh@skku.edu
- KMID: 2212064
- DOI: http://doi.org/10.3341/jkos.2009.50.2.285
Abstract
-
PURPOSE: To provide quantitative data on the distribution of MyHCeom and compare the proportion of myosin heavy chain (MyHC) isoforms between the central and peripheral regions of human extraocular muscles (EOMs).
METHODS
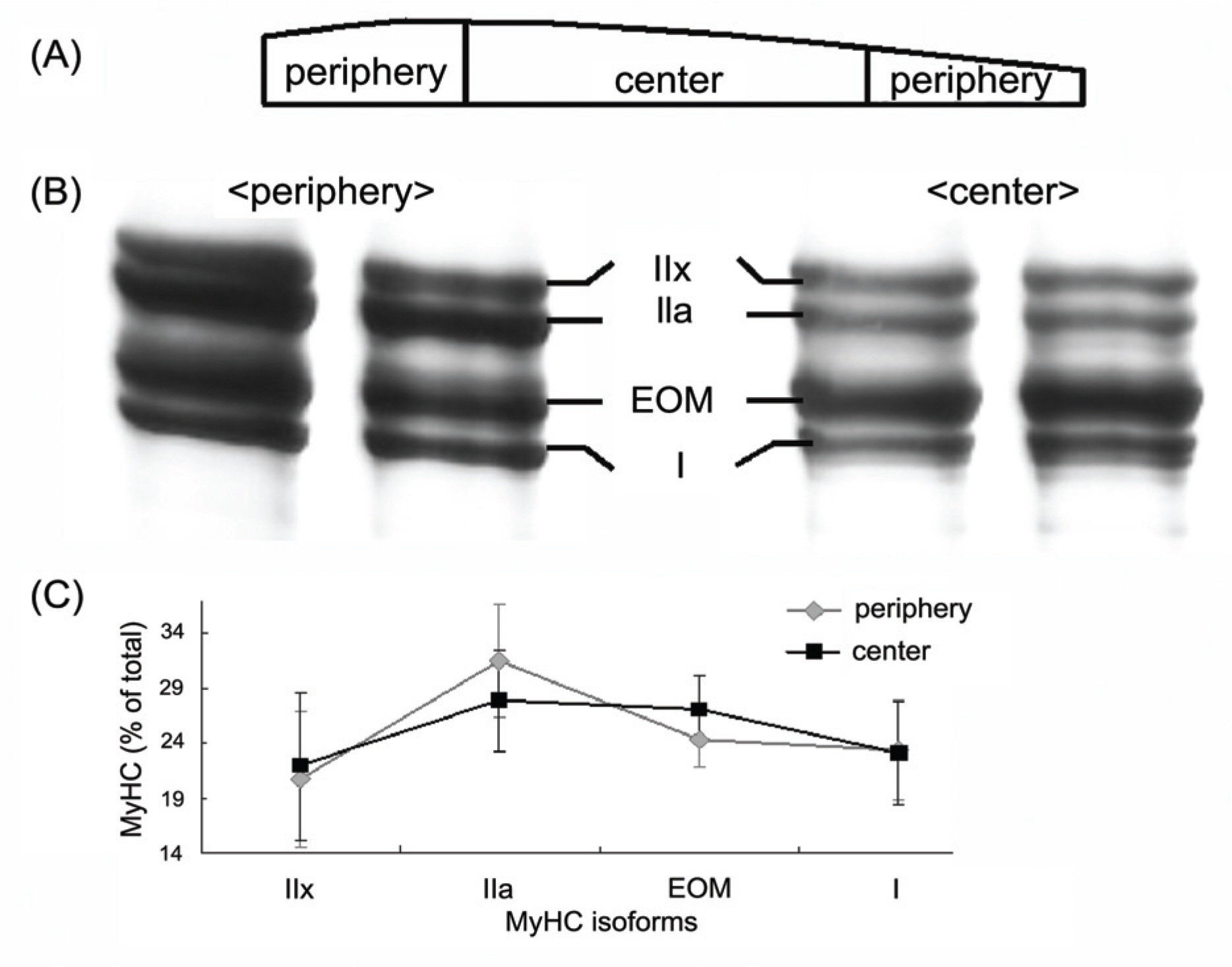
Medial rectus, lateral rectus, superior rectus, inferior rectus, superior oblique, and inferior oblique muscle samples were taken from three men with brain death. To examine the longitudinal distribution of myosin isoforms, the muscles were divided into central and peripheral portions of equal length. Electrophoresis and densitometry were used to quantify the distribution of MyHC isoforms.
RESULTS
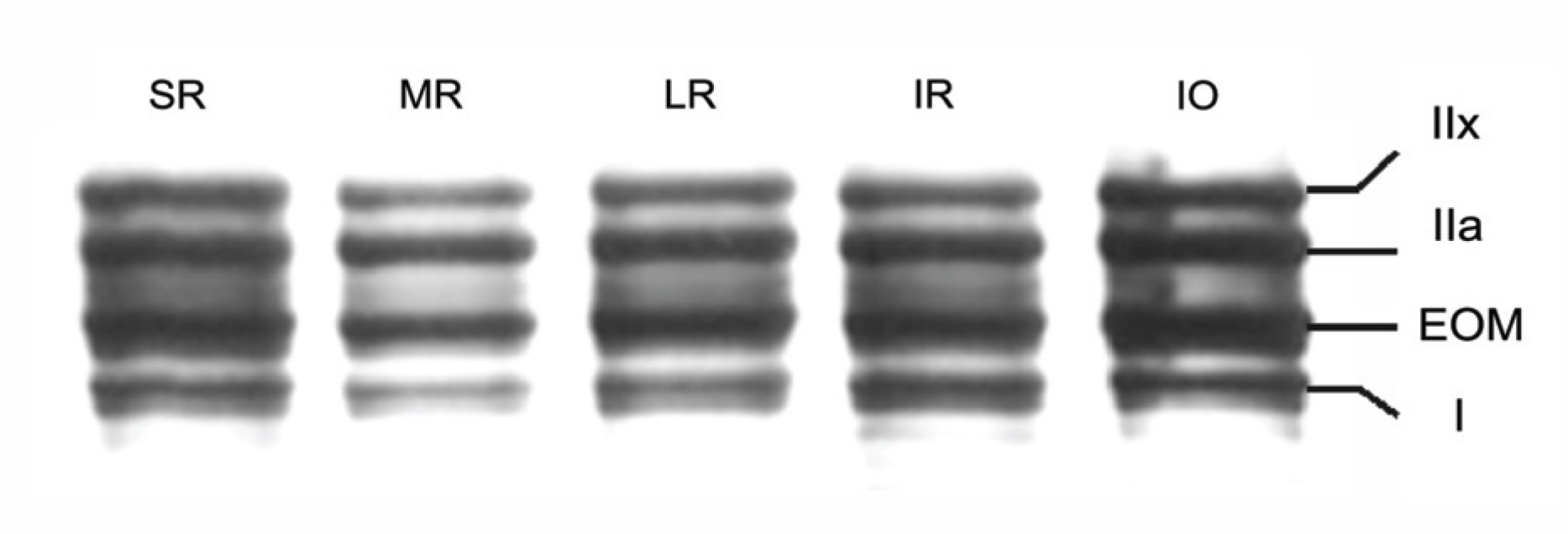
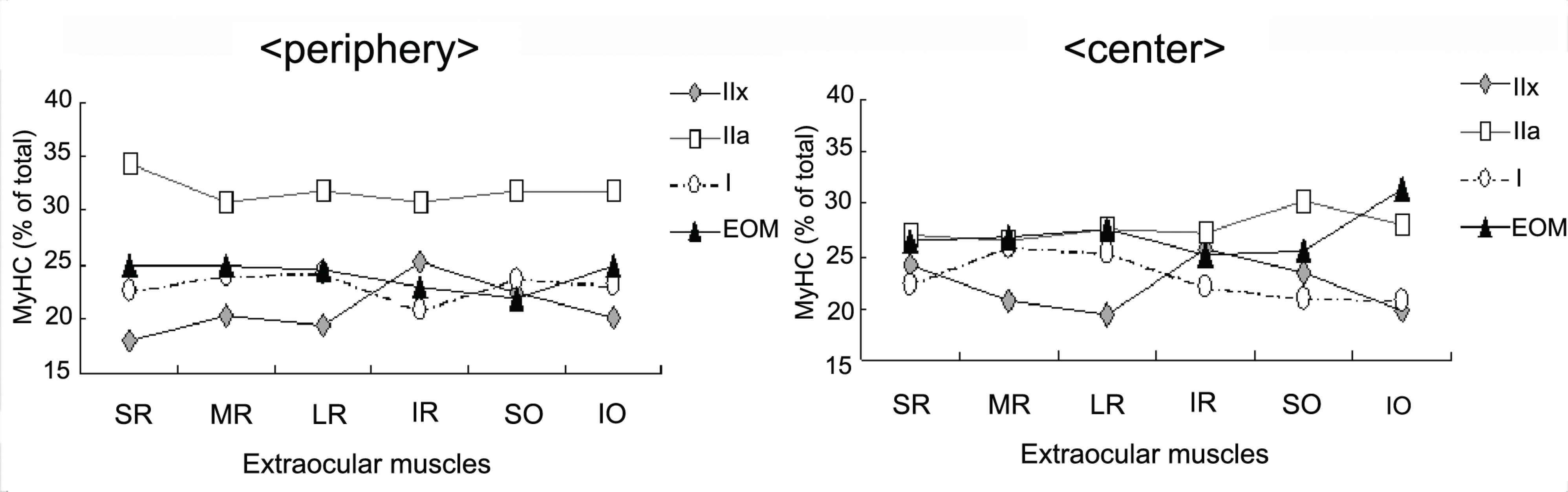
Electrophoresis of whole-muscle extracts of sampled EOMs revealed four MyHC bands that were identified as MyHCI, MyHCeom, MyHCIIa, and MyHCIIx. The proportion of MyHCeom was higher in the central region, whereas the proportion of MyHCIIa was higher in the peripheral region. The relative proportions of MyHCI, MyHCeom, and MyHCIIa were not significantly different among the EOMs. There was a tendency for higher levels of MyHCIIx in the inferior rectus muscle.
CONCLUSIONS
The proportion of MyHCeom was higher in the central region of human EOMs. Further studies are needed to investigate the consequences of this distributional difference on the function of EOMs.
MeSH Terms
Figure
Cited by 1 articles
-
The Changes in Myosin Heavy Chain Isoforms After Extraocular Muscle Recession in Rabbits
Ho-Seok Sa, Sei Yeul Oh
J Korean Ophthalmol Soc. 2009;50(8):1259-1265. doi: 10.3341/jkos.2009.50.8.1259.
Reference
-
References
1. Brueckner JK, Itkis O, Porter JD. Spatial and temporal patterns of myosin heavy chain expression in developing rat extraocular muscle. J Muscle Res Cell Motil. 1996; 17:297–312.
Article2. Jacoby J, Ko K, Weiss C, Rushbrook JI. Systematic variation in myosin expression along extraocular muscle fibers of the adult rat. J Muscle Res Cell Motil. 1990; 11:25–40.3. Rushbrook JI, Weiss C, Ko K, et al. Identification of alpha-cardiac myosin heavy chain mRNA and protein in extraocular muscle of the adult rabbit. J Muscle Res Cell Motil. 1994; 15:505–15.
Article4. Wieczorek DF, Periasamy M, Butler-Browne GS, et al. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985; 101:618–29.
Article5. Briggs MM, Schachat F. Early specialization of the superfast myosin in extraocular and laryngeal muscles. J Exp Biol. 2000; 203:2485–94.
Article6. Schachat F, Briggs MM. Phylogenetic implications of the superfast myosin in extraocular muscles. J Exp Biol. 2002; 205:2189–201.
Article7. Li ZB, Rossmanith GH, Hoh JF. Cross-bridge kinetics of rabbit single extraocular and limb muscle fibers. Invest Ophthalmol Vis Sci. 2000; 41:3770–4.8. Briggs MM, Schachat F. The superfast extraocular myosin (MYH13) is localized to the innervation zone in both the global and orbital layers of rabbit extraocular muscle. J Exp Biol. 2002; 205:3133–42.
Article9. Pedrosa-Domellöf F, Gohlsch B, Thornell LE, Pette D. Electro-phoretically defined myosin heavy chain patterns of single human muscle spindles. FEBS Lett. 1993; 335:239–42.
Article10. McLoon LK, Rios L, Wirtschafter JD. Complex three dimen-sional patterns of myosin isoform expression: differences between and within specific extraocular muscles. J Muscle Res Cell Motil. 1999; 20:771–83.11. Wasicky R, Ziya-Ghazvini F, Blumer R, et al. Muscle fiber types of human extraocular muscles: a histochemical and immunohistochemical study. Invest Ophthalmol Vis Sci. 2000; 41:980–90.12. Rubinstein NA, Hoh JF. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci. 2000; 41:3391–8.13. Kranjc BS, Sketelj J, Albis AD, et al. Fibre types and myosin heavy chain expression in the ocular medial rectus muscle of the adult rat. J Muscle Res Cell Motil. 2000; 21:753–61.14. Brueckner JK, Porter JD. Visual system maldevelopment disrupts extraocular muscle-specific myosin expression. J Appl Physiol. 1998; 85:584–92.15. Kranjc BS, Sketelj J, d'Albis A, Erzen I. Long-term changes in myosin heavy chain composition after botulinum toxin a injection into rat medial rectus muscle. Invest Ophthalmol Vis Sci. 2001; 42:3158–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Changes of Myosin Heavy Chain Isoforms after Tenotomy on Extraocular Muscle Layers of Rabbits
- The Changes in Myosin Heavy Chain Isoforms After Extraocular Muscle Recession in Rabbits
- The Effect of Sprint and Heavy Resistance Training on Muscle Strength, Endurance and Muscle Fiber Type
- The Change of Extraocular Muscle Layers After Tenotomy: Histologic and Immunohistochemical study
- Quantitative Analysis of Myosin Heavy Chain Expression Change in Laryngeal Muscle after Irradiation in Rats