J Korean Ophthalmol Soc.
2009 Feb;50(2):219-226. 10.3341/jkos.2009.50.2.219.
Short-term Safety and Efficacy of Intravitreal Bavacizumab Injection
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Pusan National University, Pusan, Korea. jlee@pusan.ac.kr
- KMID: 2212050
- DOI: http://doi.org/10.3341/jkos.2009.50.2.219
Abstract
-
PURPOSE: To evaluate the short-term safety and efficacy of intravitreal bevacizumab (Avastin(R)) injection for various conditions.
METHODS
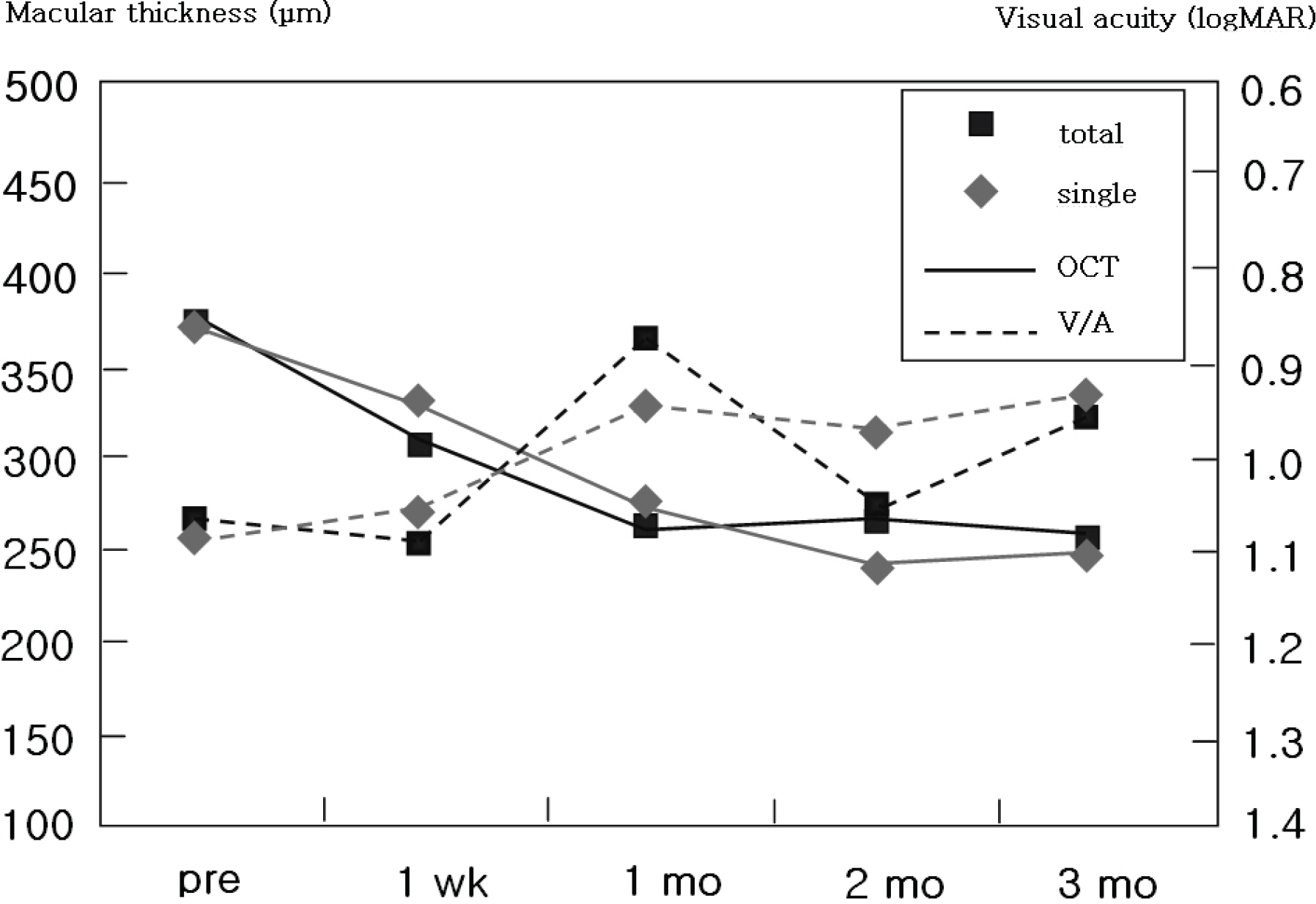
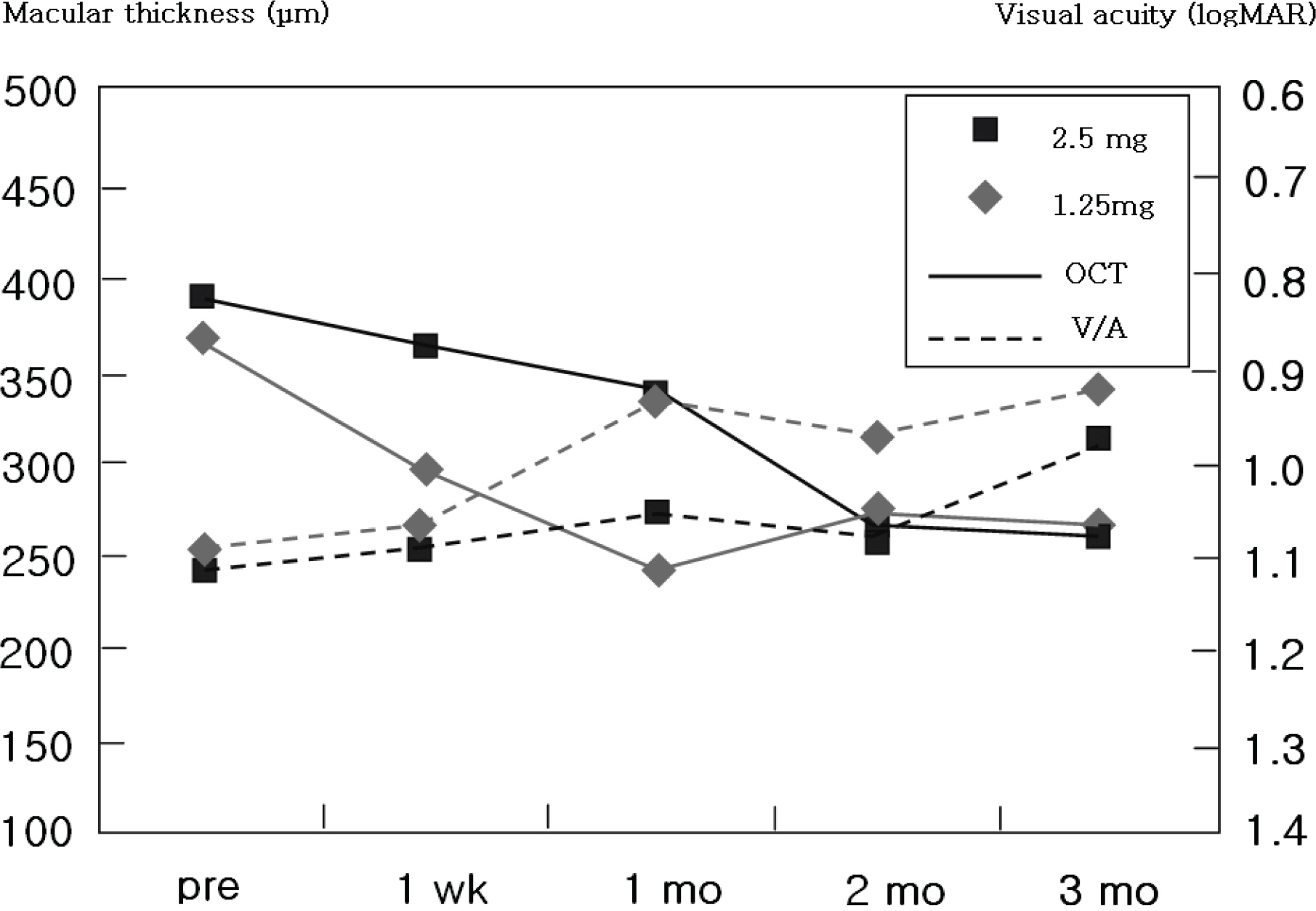
The medical records of 257 eyes of 251 patients who underwent intravitreal bevacizumab injections were reviewed. Central retinal thickness on optical coherence tomography and visual acuity before injections, at 1 week, 1 month, 2 months and 3 months after injections were analyzed.
RESULTS
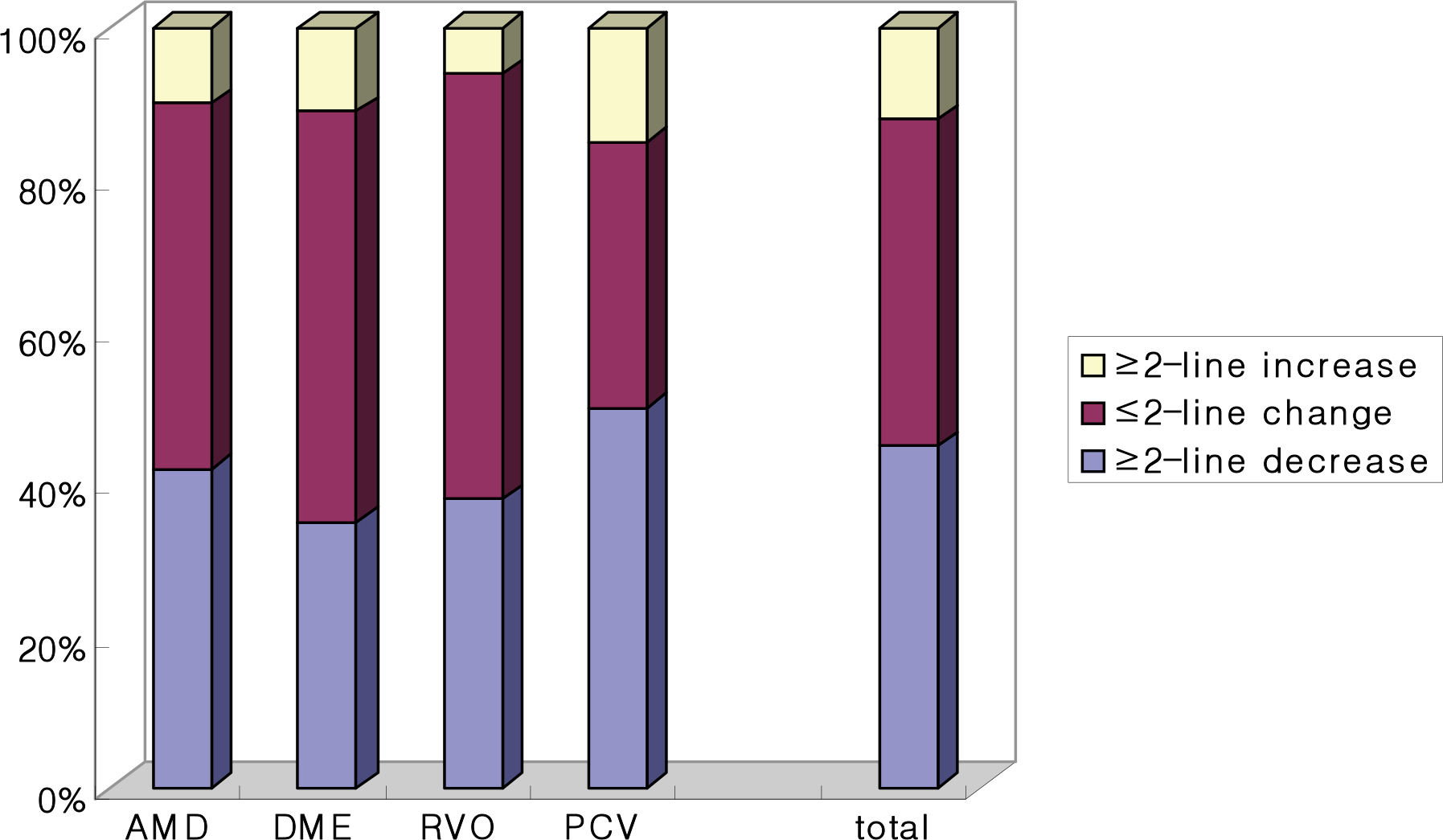
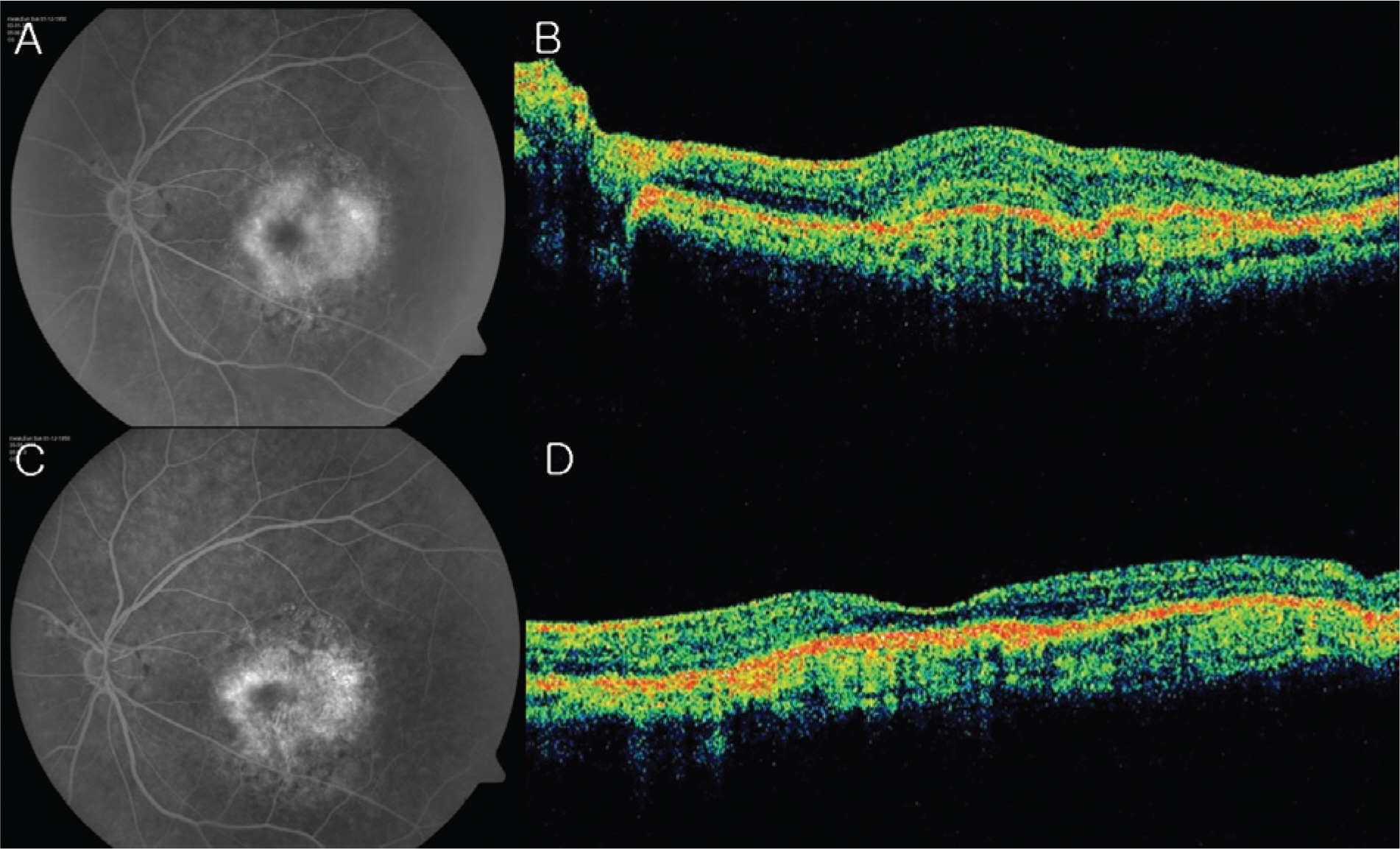
The patients included age-related macular degeneration (89 eyes), diabetic macular edema (67 eyes) and retinal vascular occlusion (57 eyes). The number of injections was twice in 82 eyes, 3 times in 23 eyes and 4 times in 2 eyes. In total, 391 injections were performed. Best corrected visual acuity increased significantly at 3 months (p=0.033) and central retinal thickness decreased by 1 month and was maintained until 3 months after the first injection (p<0.001). No serious drug-related ocular or systemic adverse incidents including endophthalmitis, glaucoma, retinal detachment, hypertension or myocardial infarction were identified.
CONCLUSIONS
The intravitreal bevacizumab injection was safe and efficient for macular edema in this short-term study.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The Long-Term Observation of Intraocular Pressure after Intravitreal Injections of Bevacizumab, Triamcinolone, and Combination of Both
Yong Woon Shin, Hee Yoon Cho, Yoon Jung Lee, Min Chul Seong
J Korean Ophthalmol Soc. 2011;52(5):574-581. doi: 10.3341/jkos.2011.52.5.574.The Short-Term Efficacy of Intravitreal Ranibizumab in the Treatment of Diabetic Macular Edema
Seok Joon Kong, Ji Wook Yang, Dong Hyun Jee
J Korean Ophthalmol Soc. 2010;51(11):1453-1458. doi: 10.3341/jkos.2010.51.11.1453.
Reference
-
References
1. Senger DR, Connolly DT, Van de Water L, et al. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990; 50:1774–8.2. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1977; 18:4–25.
Article3. Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996; 37:1929–34.
Article4. Kliffen M, Sharma HS, Mooy CM, et al. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997; 81:154–62.
Article5. Gragoudas ES, Adamis AP, Cunningham ET Jr, et al. Pegaptanib for neovascular age-related macular defeneration. N Engl J Med. 2004; 351:2805–16.6. Boyer DS, Antoszyk AN, Awh CC, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007; 114:246–52.
Article7. Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: Phase III clinical trial results. Ophthalmol Clin North Am. 2006; 19:361–72.8. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.
Article9. Gordon MS, Cunnigham D. Managing patients treated with bavacizumab combination therapy. Oncology. 2005; 69:25–33.10. Skillings JR, Johnson DH, Miller K, et al. Arterial throm-boembolic events (ATEs) is a pooled analysis of 5 randomized, controlled trials (RCTs) of bevacizumab (BV) with chemotherapy. J Clin Oncol. 2005; 23:3019.11. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherencetomography findings after an intravitreal injection of bavacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–5.12. Rosenfeld PJ, Fung AE, Puliafito VA. Optical coherence tomography findings after an intravitreal injection of bavacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005; 36:336–9.13. Rich RM, Rosenfeld PJ, Puliato CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (avastin) for neovascular age-related macular degeneration. Retina. 2006; 26:495–511.
Article14. Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006; 26:383–90.
Article15. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.
Article16. Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab (avastin) for central and hemicentral retinal vein occlusions: IBeVO study. Retina. 2007; 27:141–9.17. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007; 114:743–50.18. Fung AE, Rosenfeld PJ, Reichel E. The international intravitreal bevacizumab safety survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006; 90:1344–49.
Article19. Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999; 27:536–44.
Article20. Ferrara N, Hillan KJ, Novotony W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005; 333:328–35.
Article21. Han DP. Intravitreal human immune globulin in a rabbit model of Staphylococcus aureus tozin-mediated endophthalmitis:a potential adjunct in the treatment of endophthalmitis. Trans Am Ophthalmol Soc. 2004; 102:305–20.22. Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005; 112:1035–47.23. Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.24. Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Primary Intravitreal Ranibizumab Injection for Treatment of Type 1 Retinopathy of Prematurity
- Short-term Safety Evaluation of Resident-performed Intravitreal Injection
- Safety Evaluation of Bilateral Same-day Intravitreal Injections of Bevacizumab
- Effect of Primary Intravitreal Bevacizumab Injection on Stage 3 Retinopathy of Prematurity with Plus Signs
- Short-term Efficacy of Intravitreal Bavacizumab for Polypoidal Choroidal Vasculopathy