Short-term Efficacy of Intravitreal Bavacizumab for Polypoidal Choroidal Vasculopathy
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University Hospital, College of Medicine, Pusan, Korea. bsoum@pusan.ac.kr
- KMID: 2211897
- DOI: http://doi.org/10.3341/jkos.2009.50.1.51
Abstract
- PURPOSE
To evaluate the short-term effect of intravitreal bevacizumab (Avastin(R)) in polypoidal choroidal vasculopathy.
METHODS
Intravitreal Avastin(R) was injected into 13 eyes of 13 patients with PCV in this retrospective, interventional case study. The follow-up period lasted over 3 months after therapy. Changes in best-corrected visual acuity (BCVA), foveal height determined by optical coherence tomography, and abnormal vasculature in indocyanine green angiography (ICGA) were evaluated.
RESULTS
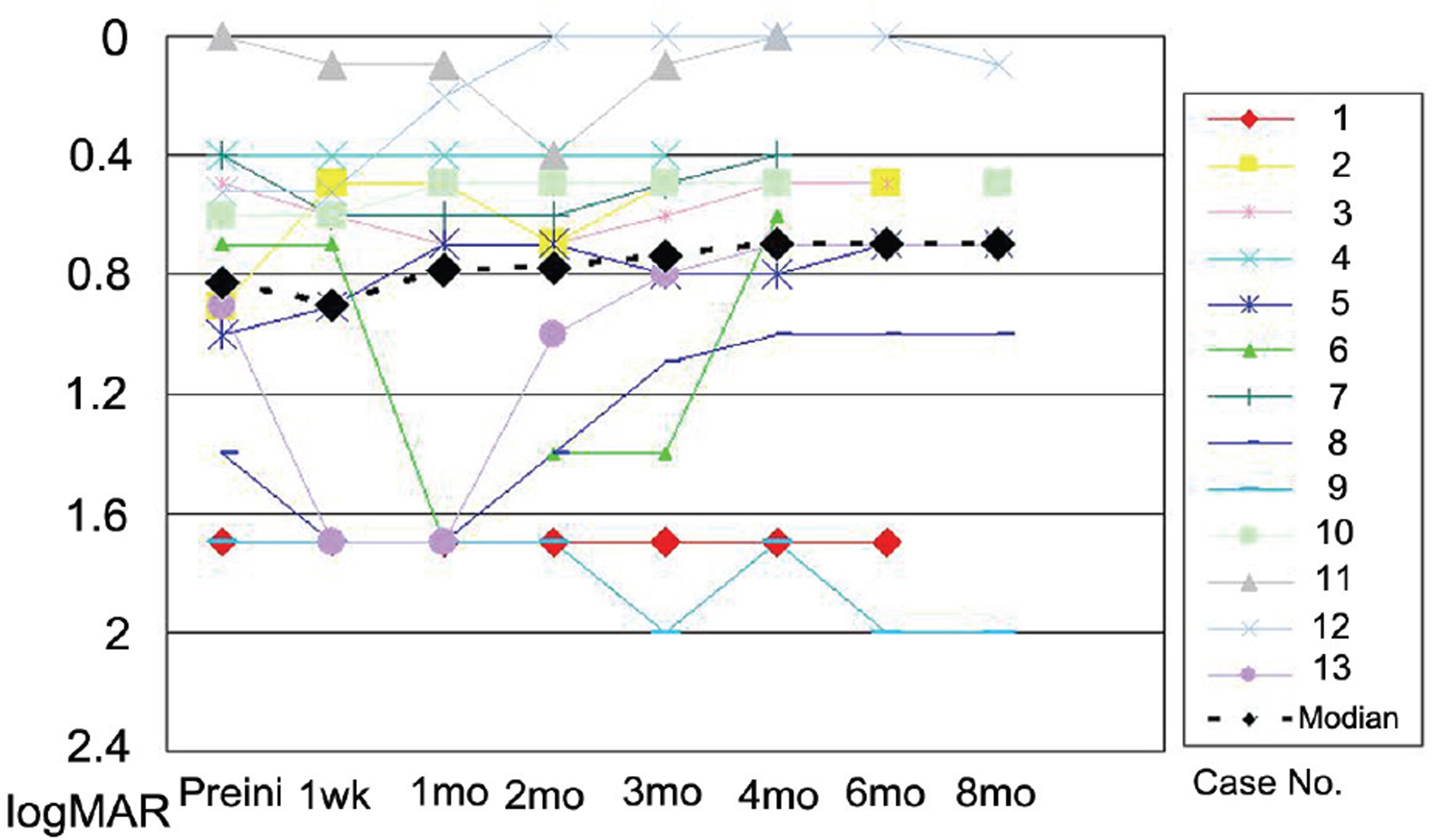
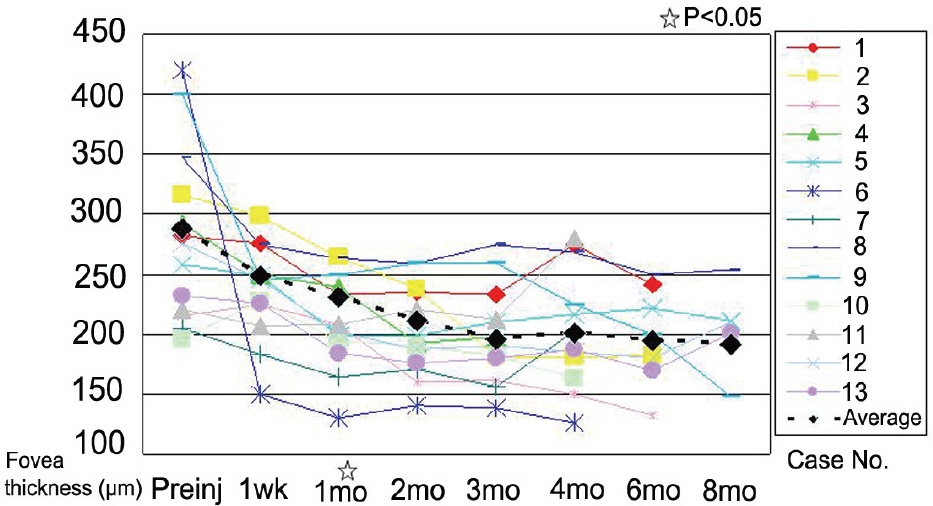
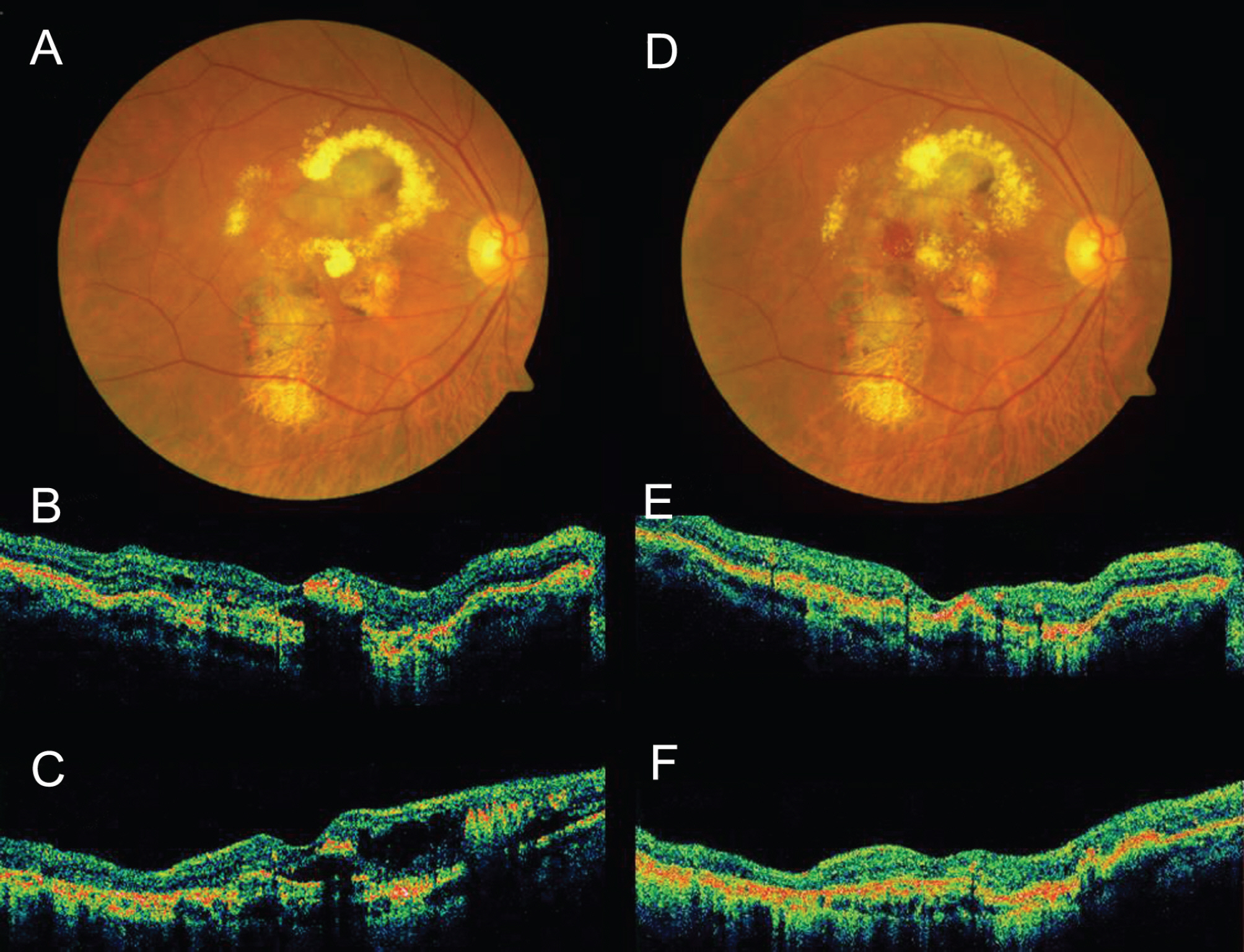
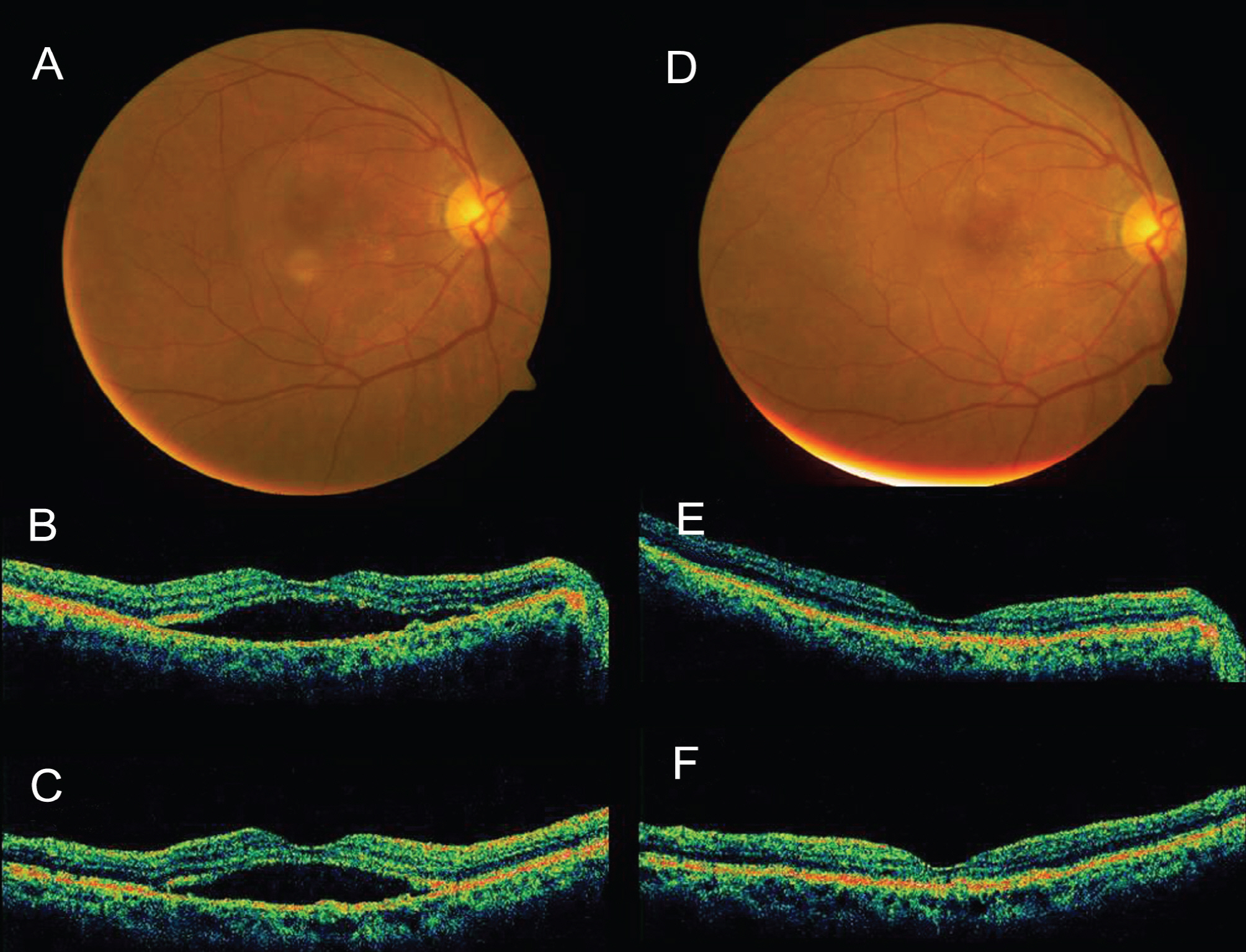
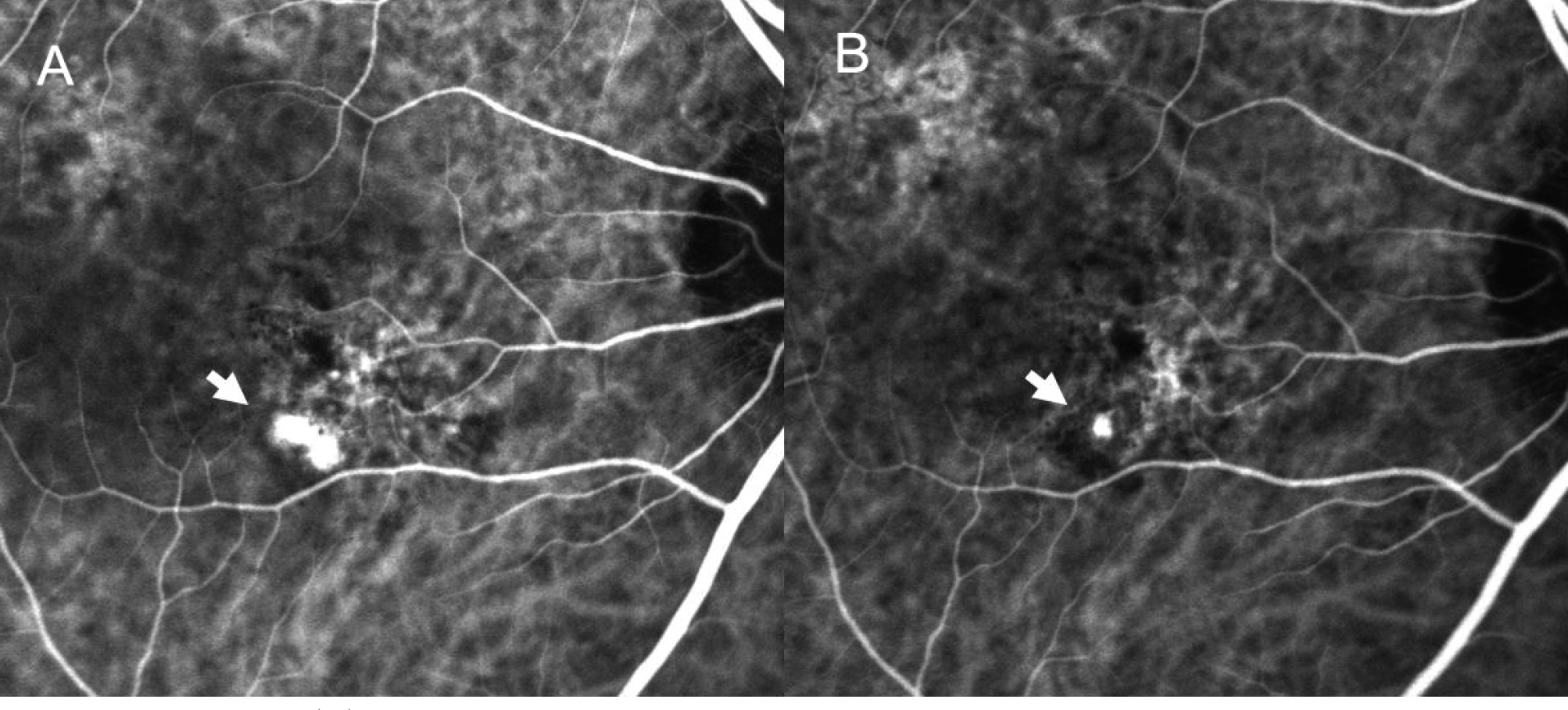
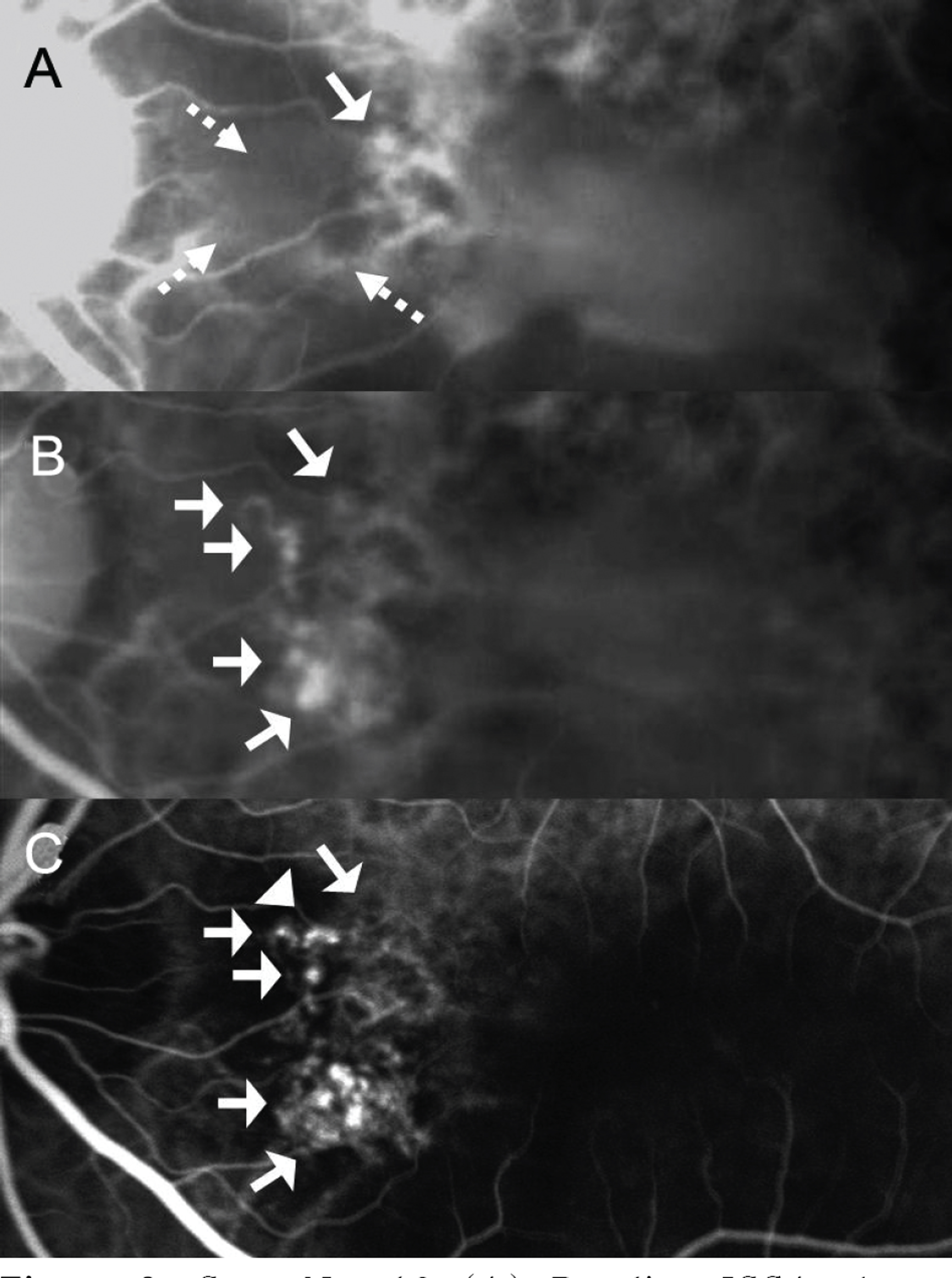
The mean LogMAR BCVA was 0.82 at baseline, 0.78 at 1 month after treatment, and 0.73 at 3 months after treatment. Visual acuity was stabilized or improved in 13 eyes (100%). The mean foveal height was 288 micrometer at baseline, 231 micrometer (p<0.05) at 1 month after treatment, and 196 micrometer at 3 months after treatment. The polypoidal lesions in ICGA decreased in 4 eyes (31%), although branching vasculature in ICGA was unchanged in 13 eyes (100%).
CONCLUSIONS
Intravitreal injection of Avastin(R) may stabilize visual acuity and reduce macular edema due to decreased retinal pigment epithelial detachment and leaking. However, intravitreal injection had a minimal effect in occlusion of the symptomatic polypoidal lesions and no effect in occlusion of the branching vascular network.
MeSH Terms
Figure
Cited by 3 articles
-
Long-term Recurrence in Neovascular Age-related Macular Degeneration or Polypoidal Choroidal Vasculopathy without First Year Recurrence
Kee Sun Tae, Jong Woo Kim, Chul Gu Kim, Dong Won Lee, Jae Hui Kim
J Korean Ophthalmol Soc. 2018;59(10):908-914. doi: 10.3341/jkos.2018.59.10.908.Electrophysiological and Morphological Changes After Intravitreal Bevacizumab Injection with Macular Edema or Choroidal Neovascularization
Hyun Joon Lee, Joo Youn Park, Young-Hoon Ohn
J Korean Ophthalmol Soc. 2009;50(12):1824-1830. doi: 10.3341/jkos.2009.50.12.1824.Long-term Treatment Outcome of Intravitreal Aflibercept Monotherapy for Polypoidal Choroidal Vasculopathy
Ye Ji Kim, Sang Yun Han, Jong Woo Kim, Chul Gu Kim, Dong Won Lee, Jae Hui Kim
J Korean Ophthalmol Soc. 2018;59(3):238-245. doi: 10.3341/jkos.2018.59.3.238.
Reference
-
References
1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.
Article2. Ciardella AP, Donsoff IM, Huang SJ. . Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004; 49:25–37.
Article3. Uyama M, Matsubara T, Fukushima I. . Idiopathic polypoidal vasculopathy in Japanese patients. Arch Ophthalmol. 1999; 117:1035–42.4. Uyama M, Wada M, Nagai Y. . Polypoidal choroidal vasculopathy: Natural history. Am J Ophthalmol. 2002; 133:639–48.5. Lee WK, Kwon SI. Polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2000; 42:2573–84.6. Scassellati-Sforzolini B, Mariotti C, Bryan R. . Polypoidal choroidal vasculopathy in Italy. Retina. 2001; 21:121–5.
Article7. Lafaut BA, Leys AM, Snyers B. . Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000; 238:752–9.
Article8. Wen F, Chen C, Wu D, Li H. Polypoidal choroidal vasculopathy in elderly Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2004; 242:625–9.
Article9. Sho K, Takahashi K, Yamada H. . Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121:1392–6.10. Lee JW, Kim IT. Epidemiologic and clinical characteristics of polypoidal choroidal vasculopathy in Korean patients. J Korean Ophthalmol Soc. 2006; 48:63–74.11. Rosa RH Jr. Davis JL, Eifrig CW. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002; 120:502–8.12. Kuroiwa S, Tatiwa H, Hisatomi T. . Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Experiment Ophthalmol. 2004; 32:297–302.
Article13. Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2005; 89:602–7.
Article14. Tong JP, Chan WM, Liu DT. . Aqueous humor levels of vascular endothelial growth factor and pigment epitheliumderived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006; 141:456–62.
Article15. Matsuoka M, Ogata N, Otsugi T. . Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004; 88:809–15.
Article16. Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003; 47:379–84.
Article17. Gomi F, Ohji M, Sayanagi K. . 1-Year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008; 115:141–6.18. Lee PY, Kim KS, Lee WK. Photodynamic therapy with verteporfin in polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2004; 45:216–27.19. Rich RM, Rosenfeld PJ, Puliaflilo GA. . Short term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006; 26:495–511.20. Spaide RF, Laud K, Fine HF. . Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006; 26:383–90.
Article21. Lee SC, Seong YS, Kim SS. . Photodynamic therapy with vertepofin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004; 218:193–201.22. Chan WM, Lam DC, Lai TY. . Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy. Ophthalmology. 2004; 111:1579–84.
Article23. Silva RM, Figueira J, Cachulo ML. . Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefe Arch Clin Exp Ophthalmol. 2005; 243:973–9.
Article24. Iijima H, Iida T, Imai M. . Optical coherence tomography of orange-red subretinal lesions in eyes with idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2000; 129:21–6.
Article25. Yang JC, Haworth L, Sherry RM. . A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003; 349:427–34.
Article26. Hurwitz H, Fehrenbacher L, Novotny W. . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.
Article27. Ghajarnia M, Kurup S, Eller A. The therapeutic effects of intravitreal bevacizumab in a patient with recalcitrant idiopathic polypoidal choroidal vasculopathy. Semin Ophthalmol. 2007; 22:127–31.
Article28. Gomi F, Sawa M, Sakaguchi H. . Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol; 2008; 92:70–3.
Article29. Shahar J, Avery RL, Heilweil G. . Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina. 2006; 26:262–9.
Article30. Heiduschka P, Julien S, Hofmeister S. . Bevacizumab (avastin) dose not harm retinal function after intravitreal injection as shown by electroretinography in adult mice. Retina. 2008; 28:46–55.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Short-Term Efficacy of Intravitreal Aflibercept for Polypoidal Choroidal Vasculopathy
- Long-term Treatment Outcome of Intravitreal Aflibercept Monotherapy for Polypoidal Choroidal Vasculopathy
- Initial Factors Associated with Resistance to Intravitreal Aflibercept Injection in Polypoidal Choroidal Vasculopathy
- Association between Vortex Vein Engorgement and Treatment Outcomes of Intravitreal Aflibercept for Polypoidal Choroidal Vasculopathy
- Analysis of Efficacy of Intravitreal Aflibercept According to Subfoveal Choroidal Thickness in Polypoidal Choroidal Vasculopathy