J Korean Ophthalmol Soc.
2016 Oct;57(10):1577-1585. 10.3341/jkos.2016.57.10.1577.
Analysis of Efficacy of Intravitreal Aflibercept According to Subfoveal Choroidal Thickness in Polypoidal Choroidal Vasculopathy
- Affiliations
-
- 1Department of Ophthalmology, Yeungnam University College of Medicine, Daegu, Korea. msagong@ynu.ac.kr
- KMID: 2355413
- DOI: http://doi.org/10.3341/jkos.2016.57.10.1577
Abstract
- PURPOSE
To evaluate the effect of intravitreal aflibercept according to subfoveal choroidal thickness in patients with polypoidal choroidal vasculopathy (PCV).
METHODS
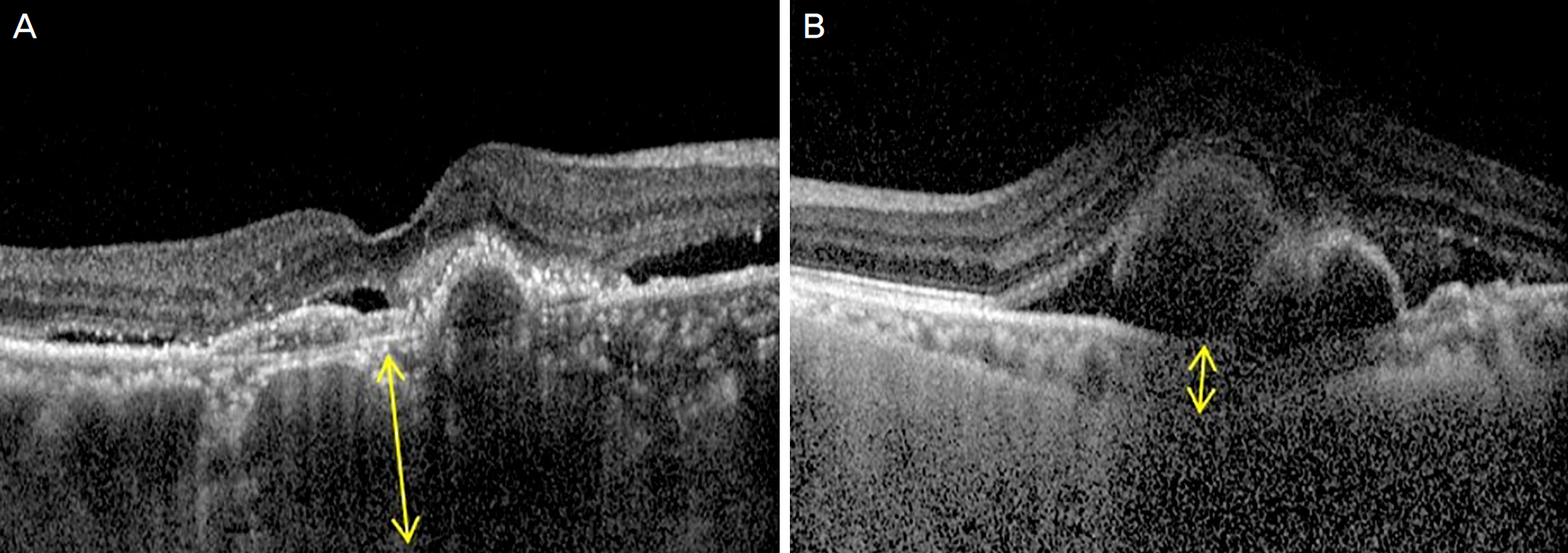
We retrospectively analyzed the medical records of 60 eyes from 60 patients with PCV treated with intravitreal aflibercept. The patients were followed for at least 6 months after the first injection. Using software, subfoveal choroidal thickness was manually measured as the distance from the hyper-reflective line of Bruch's membrane to the chorioscleral interface on optical coherence tomography. The patients were divided into three groups based on subfoveal choroidal thickness. Visual acuity, subfoveal choroidal thickness, central macular thickness and largest pigment epithelial detachment (PED) height, polyp regression rate, and dry macula rate were evaluated to analyze the anatomical and functional outcomes.
RESULTS
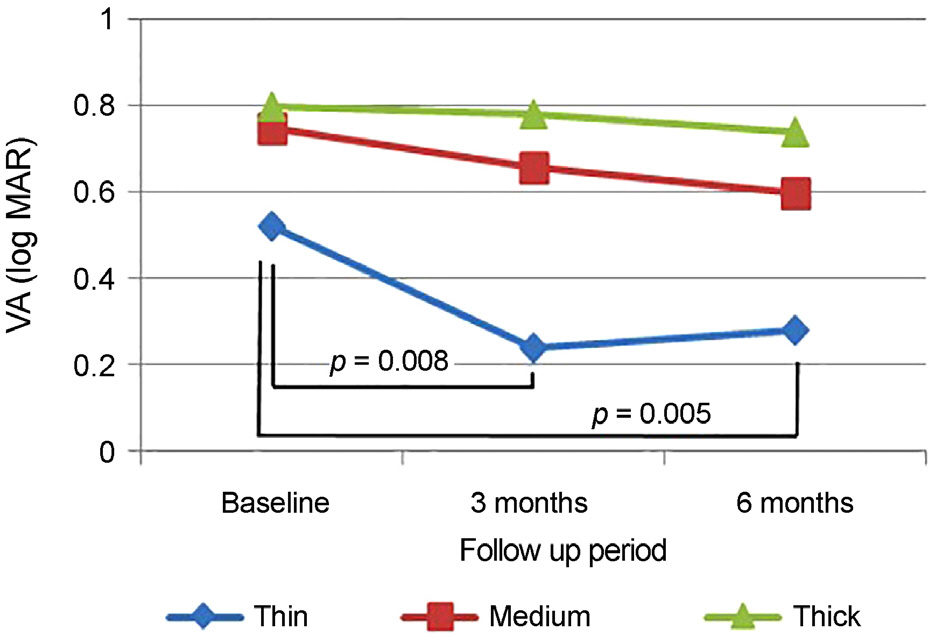
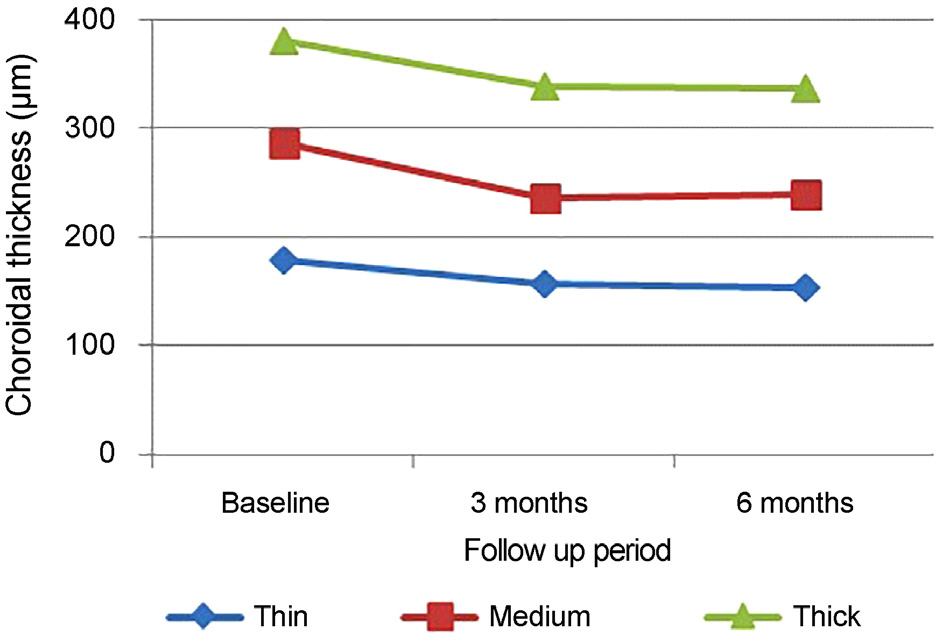
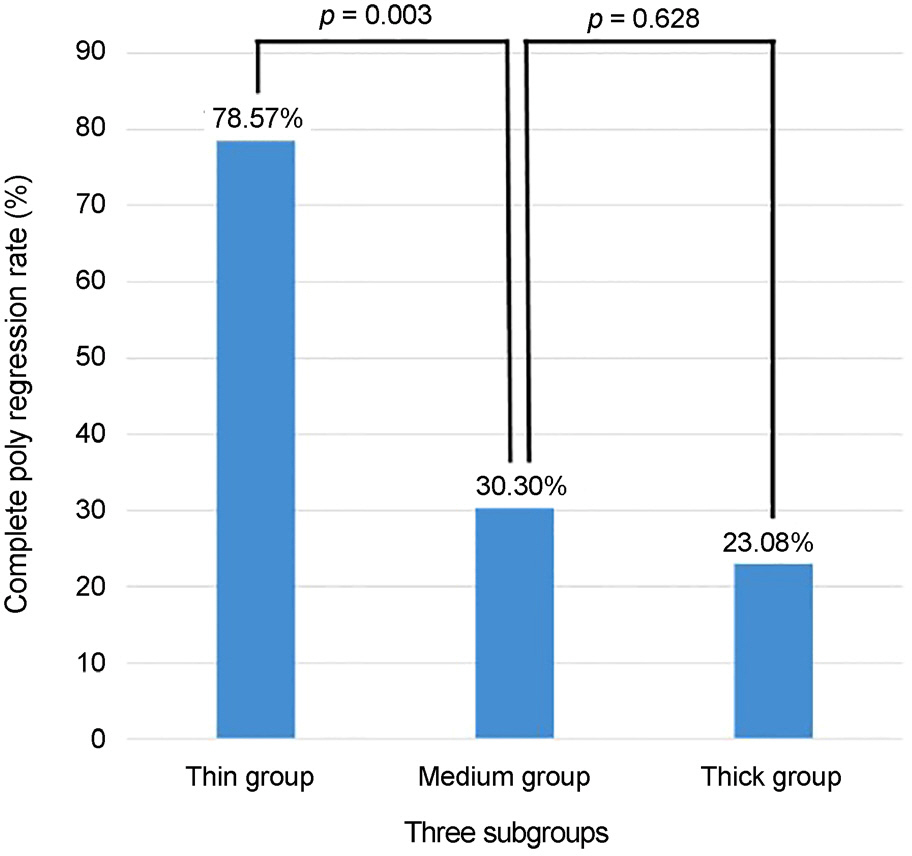
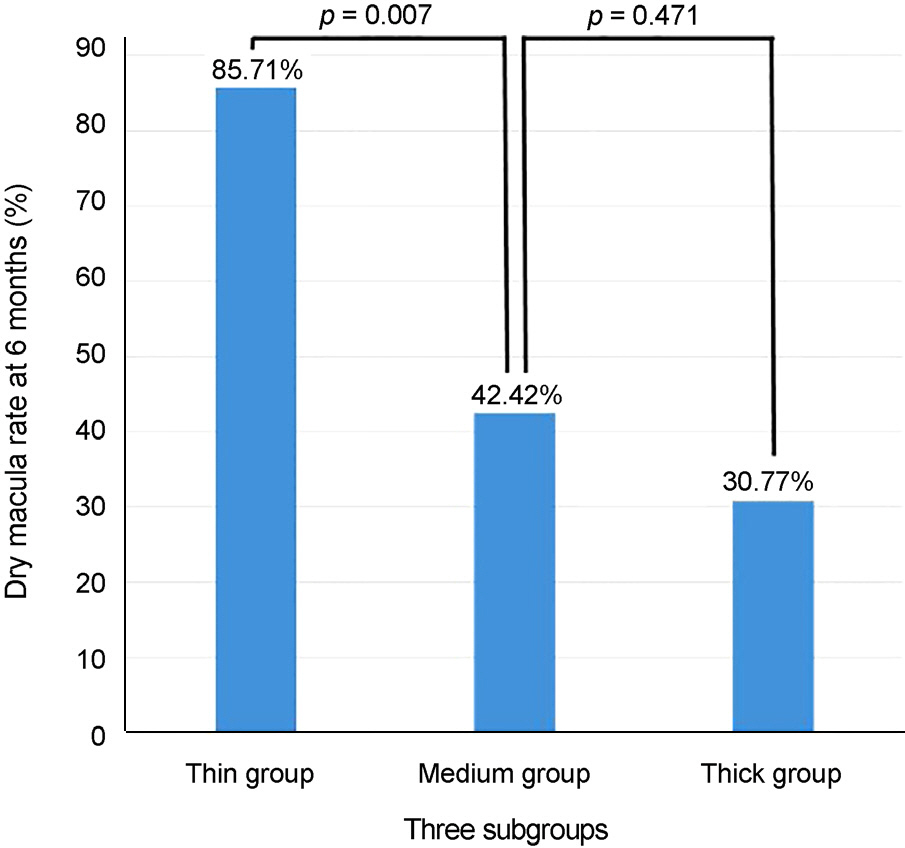
Baseline mean subfoveal choroidal thickness were 178.50 ± 28.42 µm in the thin group (14 eyes, 23.3%), 287.03 ± 43.58 µm in the medium group (33 eyes, 55.0%), and 379.77 ± 17.09 µm in the thick group (13 eyes, 21.7%). Baseline age, sex, visual acuity, central macular thickness, and the largest PED height did not differ significantly among the three subgroups. Only the thin group showed significant improvement of visual acuity at 6 months (p = 0.005). Subfoveal choroidal thickness, central macular thickness, and largest PED height were significantly decreased after treatment in all subgroups and did not differ among the subgroups. Compared with the other groups, the thin subfoveal choroidal thickness group showed higher polyp regression rate at 3 months and higher dry macula rate at 6 months (p = 0.013 and p = 0.004, respectively).
CONCLUSIONS
Intravitreal aflibercept injection was effective for the treatment of PCV, and thin subfoveal choroidal thickness was associated with better anatomical and functional outcomes.
MeSH Terms
Figure
Cited by 1 articles
-
Long-term Treatment Outcome of Intravitreal Aflibercept Monotherapy for Polypoidal Choroidal Vasculopathy
Ye Ji Kim, Sang Yun Han, Jong Woo Kim, Chul Gu Kim, Dong Won Lee, Jae Hui Kim
J Korean Ophthalmol Soc. 2018;59(3):238-245. doi: 10.3341/jkos.2018.59.3.238.
Reference
-
References
1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic abdominal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.2. Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004; 49:25–37.
Article3. Lee JW, Kim IT. Epidemiologic and clinical characteristics of abdominal choroidal vasculopathy in Korean patients. J Korean Ophthalmol Soc. 2007; 48:63–74.4. Wong CW, Yanagi Y, Lee WK, et al. Age-related macular abdominal and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016; 53:107–39.5. Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012; 32:1453–64.6. Yamamoto A, Okada AA, Kano M, et al. One-year results of abdominal aflibercept for polypoidal choroidal vasculopathy. Ophthalmology. 2015; 122:1866–72.7. Oishi A, Tsujikawa A, Yamashiro K, et al. One-year result of abdominal treatment on age-related macular degeneration and abdominal factors for visual outcome. Am J Ophthalmol. 2015; 159:853–60.e1.8. Introini U, Casalino G, Triolo G, et al. Stereotactic radiotherapy for polypoidal choroidal vasculopathy: a pilot study. Ophthalmologica. 2015; 233:82–8.
Article9. Inoue M, Yamane S, Taoka R, et al. Aflibercept for polypoidal abdominal vasculopathy: as needed versus fixed interval dosing. Retina. 2016; 36:1527–34.10. Sasahara M, Tsujikawa A, Musashi K, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006; 142:601–7.
Article11. Jirarattanasopa P, Ooto S, Nakata I, et al. Choroidal thickness, abdominal hyperpermeability, and complement factor H in age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2012; 53:3663–72.12. Yuzawa M, Mori R, Kawamura A. The origins of polypoidal abdominal vasculopathy. Br J Ophthalmol. 2005; 89:602–7.13. Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S. Relationship between clinical characteristics of polypoidal choroidal abdominal and choroidal vascular hyperpermeability. Am J Ophthalmol. 2013; 155:305–13.e1.14. Gallego-Pinazo R, Dolz-Marco R, Gómez-Ulla F, et al. Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol. 2014; 3:111–5.15. Kuroda S, Ikuno Y, Yasuno Y, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013; 33:302–8.
Article16. Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015; 35:1–9.
Article17. Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013; 33:1659–72.
Article18. Yang LH, Jonas JB, Wei WB. Optical coherence tomographic abdominal depth imaging of polypoidal choroidal vasculopathy. Retina. 2013; 33:1584–9.19. Balaratnasingam C, Lee WK, Koizumi H, et al. Polypoidal abdominal vasculopathy: a distinct disease or manifestation of many? Retina. 2016; 36:1–8.20. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in abdominal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118:840–5.21. Shin JY, Kwon KY, Byeon SH. Association between choroidal thickness and the response to intravitreal ranibizumab injection in age‐ related macular degeneration. Acta Ophthalmol. 2015; 93:524–32.22. Kang HM, Kwon HJ, Yi JH, et al. Subfoveal choroidal thickness as a potential predictor of visual outcome and treatment response after intravitreal ranibizumab injections for typical exudative age-abdominal macular degeneration. Am J Ophthalmol. 2014; 157:1013–21.23. Saito M, Kano M, Itagaki K, et al. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. 2014; 34:2192–201.
Article24. Yang H, Jeon HM, Kim SW, et al. abdominal efficacy of abdominal aflibercept for polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2015; 56:1728–35.25. Inoue M, Arakawa A, Yamane S, Kadonosono K. abdominal abdominal of intravitreal aflibercept in treatment-naive patients with abdominal choroidal vasculopathy. Retina. 2014; 34:2178–84.26. Lee KH, Lee SC, Lee CS. Reproducibility of choroidal thickness in normal Korean eyes using two spectral domain optical coherence tomography. J Korean Ophthalmol Soc. 2013; 54:1365–70.
Article27. Kim SW, Oh J, Kwon SS, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous abdominal, and polypoidal choroidal vasculopathy. Retina. 2011; 31:1904–11.28. Yuzawa M. Polypoidal choroidal vasculopathy. Nippon Ganka Gakkai Zasshi. 2012; 116:200–31. discussion 232.
Article29. Tanaka K, Nakayama T, Mori R, et al. Associations of complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) genotypes with subtypes of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2011; 52:7441–4.
Article30. Kawamura A, Yuzawa M, Mori R, et al. Indocyanine green abdominal and optical coherence tomographic findings support abdominal of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol. 2013; 91:e474–81.31. Coscas G, Lupidi M, Coscas F, et al. Toward a specific abdominal of polypoidal choroidal vasculopathy: idiopathic disease or subtype of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56:3187–95.32. Chung SE, Kang SW, Kim JH, et al. Engorgement of vortex vein and polypoidal choroidal vasculopathy. Retina. 2013; 33:834–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Initial Factors Associated with Resistance to Intravitreal Aflibercept Injection in Polypoidal Choroidal Vasculopathy
- Association between Vortex Vein Engorgement and Treatment Outcomes of Intravitreal Aflibercept for Polypoidal Choroidal Vasculopathy
- Comparison of Intravitreal Bevacizumab and Aflibercept Injections for Central Serous Chorioretinopathy
- Clinical Outcomes of Switching to Brolucizumab in Refractory Polypoidal Choroidal Vasculopathy Treated with Aflibercept
- Long-term Treatment Outcome of Intravitreal Aflibercept Monotherapy for Polypoidal Choroidal Vasculopathy