J Korean Ophthalmol Soc.
2008 Apr;49(4):634-640. 10.3341/jkos.2008.49.4.634.
The Effect of Intracameral Triamcinolone Acetonide Injection on the Cornea in Rabbits
- Affiliations
-
- 1Department of Ophthalmology, Soonchunhyang University College of Medicine, Seoul, Korea. ckseek@naver.com
- KMID: 2211496
- DOI: http://doi.org/10.3341/jkos.2008.49.4.634
Abstract
-
PURPOSE: To evaluate the safety and effects of intracameral triamcinolone acetonide injection in rabbit corneas.
METHODS
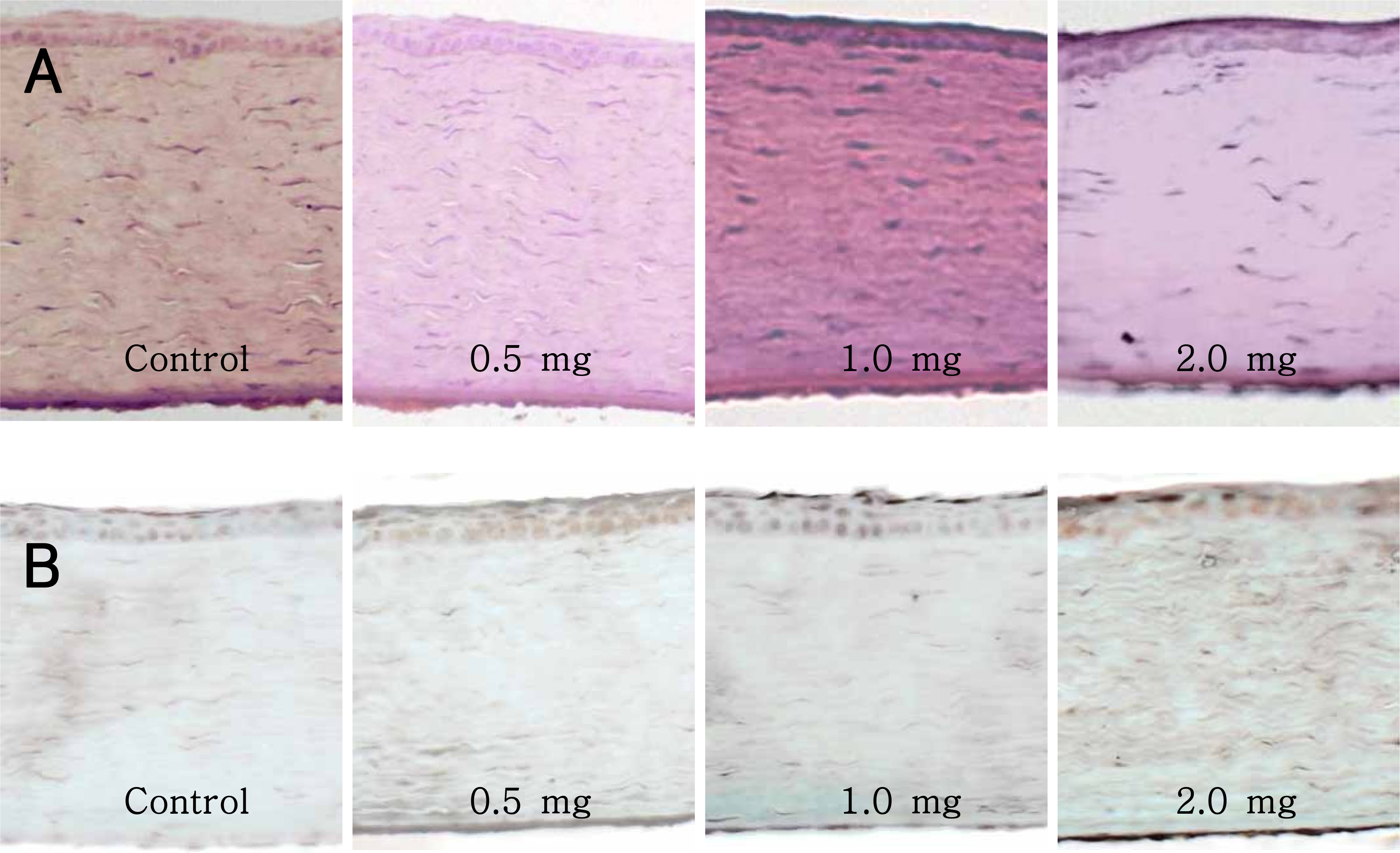
Triamcinolone acetonide in the amounts of 0.5, 1, and 2 mg was injected into the anterior chamber of rabbit eyes, and intraocular pressure, corneal thickness, and endothelial cell counts were evaluated on days 1, 3, 7, 14, and 28. Twenty-eight days after triamcinolone acetonide injection, the eyes were enucleated and examined after TUNEL staining.
RESULTS
No statistically significant differences were found among control, 0.5, and 1 mg triamcinolone-injected eyes in central corneal thickness, endothelial cell density, pleomorphism, and polymegathism. There was no difference between 2 mg triamcinolone-injected eyes and control eyes for corneal thickness and cell density, but there were statistically significant differences between these two groups for pleomorphism (p<0.05) and polymegathism (p<0.05).
CONCLUSIONS
The results of this study suggested that intracameral injections of 0.5~1 mg of triamcinolone acetonide are beneficial and cause no toxic effects on corneas.
MeSH Terms
Figure
Reference
-
References
1. Gordon DM. Prednisone and prednisolone in ocular disease. Am J Ophthalmol. 1956; 41:593–600.2. Machemer R, Sugita G, Tano Y. Treatment of intraocular proliferations with intravitreal steroids. Trans Am Ophthalmol Soc. 1979; 77:171–80.3. Kok H, Lau C, Maycock N, et al. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 2005; 112:1916.
Article4. Arevalo JF, Garcia RA, Mendoza AJ. Indocyanine green-mediated photothrombosis with intravitreal triamcinolone acetonide for subfoveal choroidal neovascularization in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2005; 243:1180–5.
Article5. Chieh JJ, Roth DB, Liu M, et al. Intravitreal triamcinolone acetonide for diabetic macular edema. Retina. 2005; 25:828–34.
Article6. Cekic O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2005; 25:851–5.
Article7. Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide for pseudophakic cystoid macular edema. Am J Ophthalmol. 2003; 136:384–6.
Article8. Oh J-Y, Wee WR, Lee JH, Kim MK. Short-term effect of intracameral triamcinolone acetonide on corneal endothelium using the rabbit model. Eye. 2007; 21:812–8.
Article9. Hernaez‐ Ortega MC, Soto‐ Pedre E. Removal of benzyl alcohol from a commercially available triamcinolone acetonide suspension for intravitreal use. Ophthalmic Surg Lasers Imaging. 2006; 37:162–4.
Article10. Jonas JB, Degenring RF, Kreissig I, et al. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005; 112:593–8.
Article11. Chan CK, Fan DS, Chan WM, et al. Ocular-hypertensive response and corneal endothelial changes after intravitreal triamcinolone injections in Chinese subjects: a 6-month follow-up study. Eye. 2005; 19:625–30.
Article12. Jonas JB, Kreissig I, Degenring RF. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87:24–7.
Article13. Vajpayee RB, Sharma N, Dada T, et al. Management of posterior capsule tears. Surv Ophthalmol. 2001; 45:473–88.
Article14. Akura J, Hatta S, Kaneda S, et al. Management of posterior capsule rupture during phacoemulsification using the dry technique. J Cataract Refract Surg. 2001; 27:982–9.
Article15. Yamakiri K, Uchino E, Kimura K, Sakamoto T. Intracameral triamcinolone helps to visualize and remove the vitreous body in anterior chamber in cataract surgery. Am J Ophthalmol. 2004; 138:650–2.
Article16. Jonas JB. Intraocular availability of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004; 137:560–2.
Article17. Chen TY, Yang CM, Liu KR. Intravitreal triamcinolone staining observation of residual undetached cortical vitreous after posterior vitreous detachment. Eye. 2006; 20:423–7.
Article18. Furino C, Micelli Ferrari T, Boscia F, et al. Triamcinolone-assisted pars plana vitrectomy for proliferative vitreoretinopathy. Retina. 2003; 23:771–6.
Article19. Walker TD. Benzalkonium toxicity. Clin Experiment Ophthalmol. 2004; 32:657.20. Morrison VL, Koh HJ, Cheng L, et al. Intravitreal toxicity of the kenalog vehicle (benzyl alcohol) in rabbits. Retina. 2006; 26:339–44.
Article21. Garcia-Arumi J, Boixadera A, Giralt J, et al. Comparison of different techniques for purification of triamcinolone acetonide suspension for intravitreal use. Br J Ophthalmol. 2005; 89:1112–4.
Article22. Nishimura A, Kobayashi A, Segawa Y, et al. Isolating triamcinolone acetonide particles for intravitreal use with a porous membrane filter. Retina. 2003; 23:777–9.
Article23. Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye. 2005; 19:1133–5.
Article24. Misra A, Burton RL. Incidence of intraoperative complications during phacoemulsification in vitrectomized and nonvitrectomized eyes: prospective study. J Cataract Refract Surg. 2005; 31:1011–4.
Article25. Mason JO 3rd, Somaiya MD, Singh RJ. Intravitreal concentration and clearance of triamcinolone acetonide in nonvitrectomized human eyes. Retina. 2004; 24:900–4.
Article26. Zinn KM. Iatrogenic intraocular injection of depot corticosteroid and its surgical removal using the pars plana approach. Ophthalmology. 1981; 88:13–7.27. Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Visual Sci. 1977; 10:597–613.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intralesional Injection of Verapamil Only and Verapamil and Serial Triamcinolone Acetonide in Peyronie's Disease
- Change of Residual Period and Clearance Rate of Intravitreal Triamcinolone According to Initial Injection Dosage
- Observation on the Effect of Triamcinolone Local Injection in Urethral Syndrome
- Repeated Local Injection of Triamcinolone Acetonide in Carpal Tunnel Syndrome
- Effect of Intracameral Triamcinolone to Control Inflammation in Rabbit Eyes