J Pathol Transl Med.
2016 Jan;50(1):52-57. 10.4132/jptm.2015.10.09.
Immunohistochemical Expression and Clinical Significance of Suggested Stem Cell Markers in Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Pathology, Research Institute of Clinical Medicine of Chonbuk National University, Biomedical Research Institute of Chonbuk National University Hospital and Research Institute for Endocrine Sciences, Chonbuk National University Medical Schoo
- KMID: 2211401
- DOI: http://doi.org/10.4132/jptm.2015.10.09
Abstract
- BACKGROUND
Increasing evidence has shown that tumor initiation and growth are nourished by a small subpopulation of cancer stem cells (CSCs) within the tumor mass. CSCs are posited to be responsible for tumor maintenance, growth, distant metastasis, and relapse after curative operation. We examined the expression of CSC markers in paraffin-embedded tissue sections of hepatocellular carcinoma (HCC) and correlated the results with clinicopathologic characteristics.
METHODS
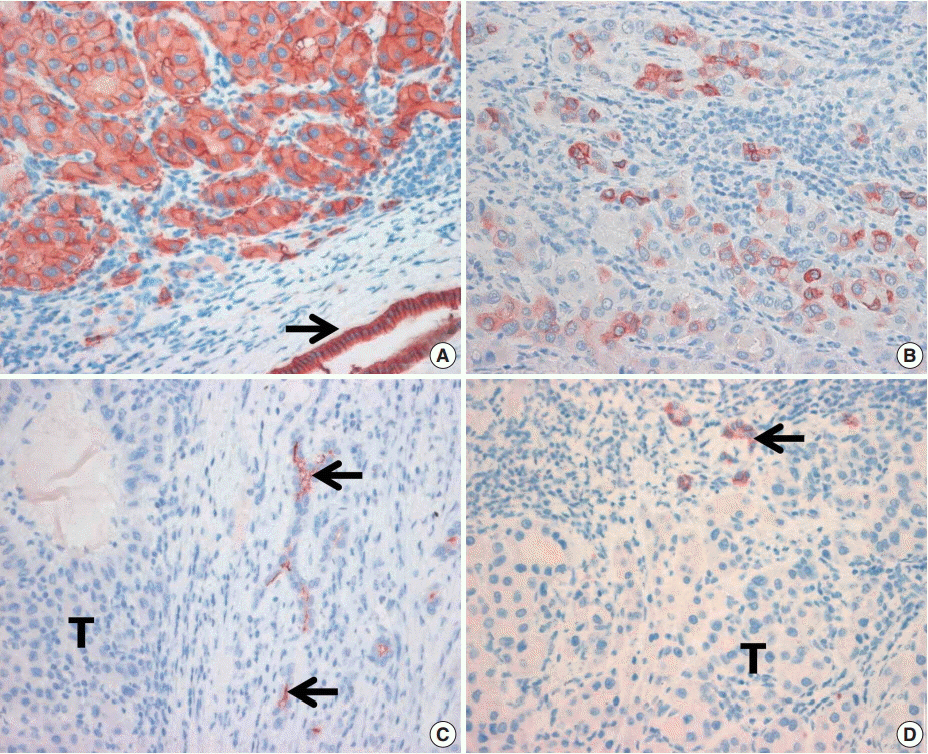
Immunohistochemical staining for the markers believed to be expressed in the CSCs, including epithelial cell adhesion molecule (EpCAM), keratin 19 (K19), CD133, and CD56, was performed in 82 HCC specimens.
RESULTS
EpCAM expression was observed in 56% of the HCCs (46/82) and K19 in 6% (5/82). EpCAM expression in HCC significantly correlated with elevated alpha-fetoprotein level, microvessel invasion of tumor cells, and high histologic grade. In addition, EpCAM expression significantly correlated with K19 expression. The overall survival and relapsefree survival rates in patients with EpCAM-expressing HCC were relatively lower than those in patients with EpCAM-negative HCC. All but two of the 82 HCCs were negative for CD133 and CD56, respectively.
CONCLUSIONS
Our results suggest that HCCs expressing EpCAM are associated with unfavorable prognostic factors and have a more aggressive clinical course than those not expressing EpCAM. Further, the expression of either CD133 or CD56 in paraffin-embedded HCC tissues appears to be rare.
MeSH Terms
Figure
Cited by 1 articles
-
Clinicopathologic Significance of Survivin Expression in Relation to CD133 Expression in Surgically Resected Stage II or III Colorectal Cancer
Wanlu Li, Mi-Ra Lee, EunHee Choi, Mee-Yon Cho
J Pathol Transl Med. 2017;51(1):17-23. doi: 10.4132/jptm.2016.09.23.
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008; 134:1752–63.
Article3. Visvader JE. Cells of origin in cancer. Nature. 2011; 469:314–22.
Article4. Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells: perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006; 66:9339–44.5. Bomken S, Fiser K, Heidenreich O, Vormoor J. Understanding the cancer stem cell. Br J Cancer. 2010; 103:439–45.
Article6. Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005; 5:899–904.7. Marquardt JU, Factor VM, Thorgeirsson SS. Epigenetic regulation of cancer stem cells in liver cancer: current concepts and clinical implications. J Hepatol. 2010; 53:568–77.
Article8. Yamashita T, Ji J, Budhu A, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009; 136:1012–24.
Article9. Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma: a possible progenitor cell origin. Histopathology. 2006; 49:138–51.
Article10. Zhu Z, Hao X, Yan M, et al. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010; 126:2067–78.11. Chan AW, Tong JH, Chan SL, Lai PB, To KF. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology. 2014; 64:935–50.
Article12. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.13. Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012; 10:717–28.
Article14. Yang XR, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010; 59:953–62.
Article15. Kim H, Choi GH, Na DC, et al. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011; 54:1707–17.
Article16. Guo Z, Li LQ, Jiang JH, Ou C, Zeng LX, Xiang BD. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J Gastroenterol. 2014; 20:2098–106.
Article17. Litvinov SV, Balzar M, Winter MJ, et al. Epithelial cell adhesion molecule (Ep-CAM) modulates cell-cell interactions mediated by classic cadherins. J Cell Biol. 1997; 139:1337–48.
Article18. Went PT, Lugli A, Meier S, et al. Frequent EpCAM protein expression in human carcinomas. Hum Pathol. 2004; 35:122–8.
Article19. Breuhahn K, Baeuerle PA, Peters M, et al. Expression of epithelial cellular adhesion molecule (Ep-CAM) in chronic (necro-)inflammatory liver diseases and hepatocellular carcinoma. Hepatol Res. 2006; 34:50–6.
Article20. Yamashita T, Forgues M, Wang W, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008; 68:1451–61.21. Govaere O, Komuta M, Berkers J, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014; 63:674–85.
Article22. Shan YF, Huang YL, Xie YK, et al. Angiogenesis and clinicopathologic characteristics in different hepatocellular carcinoma subtypes defined by EpCAM and alpha-fetoprotein expression status. Med Oncol. 2011; 28:1012–6.23. Kimura O, Kondo Y, Kogure T, et al. Expression of EpCAM increases in the hepatitis B related and the treatment-resistant hepatocellular carcinoma. Biomed Res Int. 2014; 2014:172913.
Article24. Kim H, Park YN. Hepatocellular carcinomas expressing ‘stemness’-related markers: clinicopathological characteristics. Dig Dis. 2014; 32:778–85.
Article25. Zen C, Zen Y, Mitry RR, et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transpl. 2011; 17:943–54.
Article26. Yeh CT, Kuo CJ, Lai MW, et al. CD133-positive hepatocellular carcinoma in an area endemic for hepatitis B virus infection. BMC Cancer. 2009; 9:324.
Article27. Tsuchiya A, Kamimura H, Takamura M, et al. Clinicopathological analysis of CD133 and NCAM human hepatic stem/progenitor cells in damaged livers and hepatocellular carcinomas. Hepatol Res. 2009; 39:1080–90.28. Tsuchiya A, Kamimura H, Tamura Y, et al. Hepatocellular carcinoma with progenitor cell features distinguishable by the hepatic stem/progenitor cell marker NCAM. Cancer Lett. 2011; 309:95–103.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD44 expression in patients with combined hepatocellular cholangiocarcinoma
- Stem Cell Markers Predict the Response to Sorafenib in Patients with Hepatocellular Carcinoma
- Expression of CD133, CD24, CD44 in Cutaneous Malignant Tumors
- Cell proliferation index and the expression of p53 and Bcl-2 in tumorous and non-tumorous lesions of hepatocellular carcinoma and metastatic liver cancer
- Arginase-1 and P-glycoprotein are downregulated in canine hepatocellular carcinoma