J Korean Soc Radiol.
2010 Feb;62(2):167-178. 10.3348/jksr.2010.62.2.167.
Dynamic Contrast-Enhanced MR Imaging of Renal Ischemia-Reperfusion Injury
- Affiliations
-
- 1Department of Radiology and Nuclear Medicine, The Catholic University of Korea, Korea. ami@catholic.ac.kr
- KMID: 2208964
- DOI: http://doi.org/10.3348/jksr.2010.62.2.167
Abstract
-
PURPOSE: To evaluate the usefulness of magnetic resonance imaging (MRI) in a renal ischemia-reperfusion injury.
MATERIALS AND METHODS
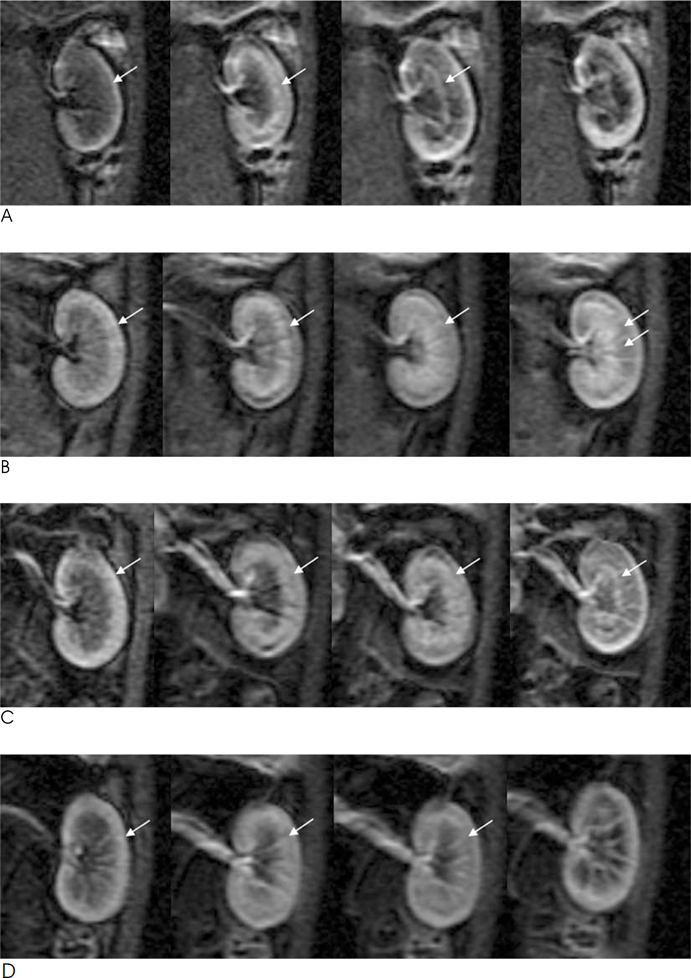
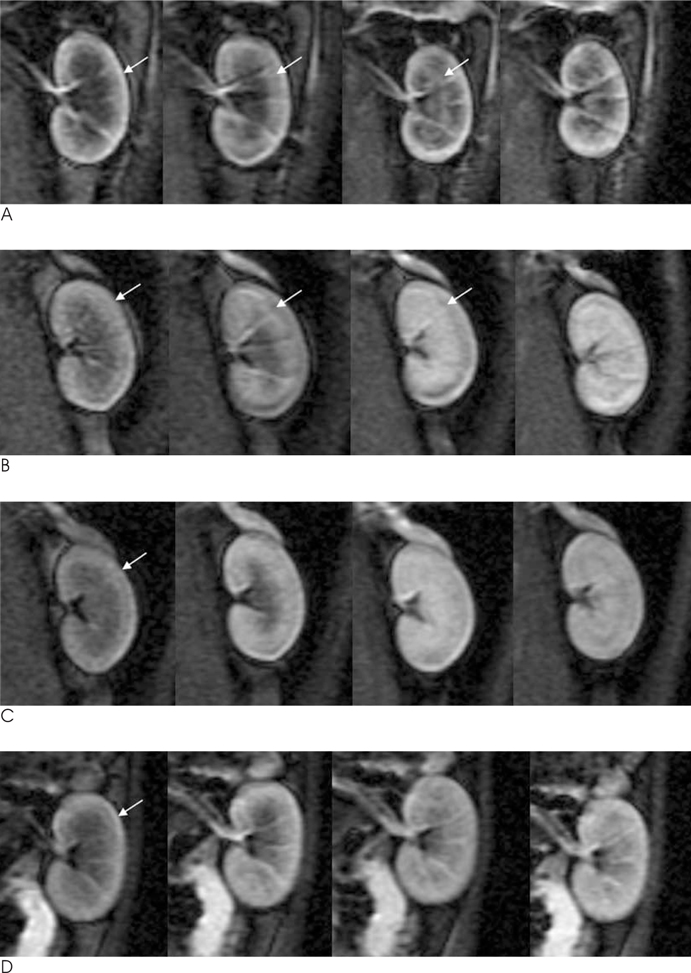
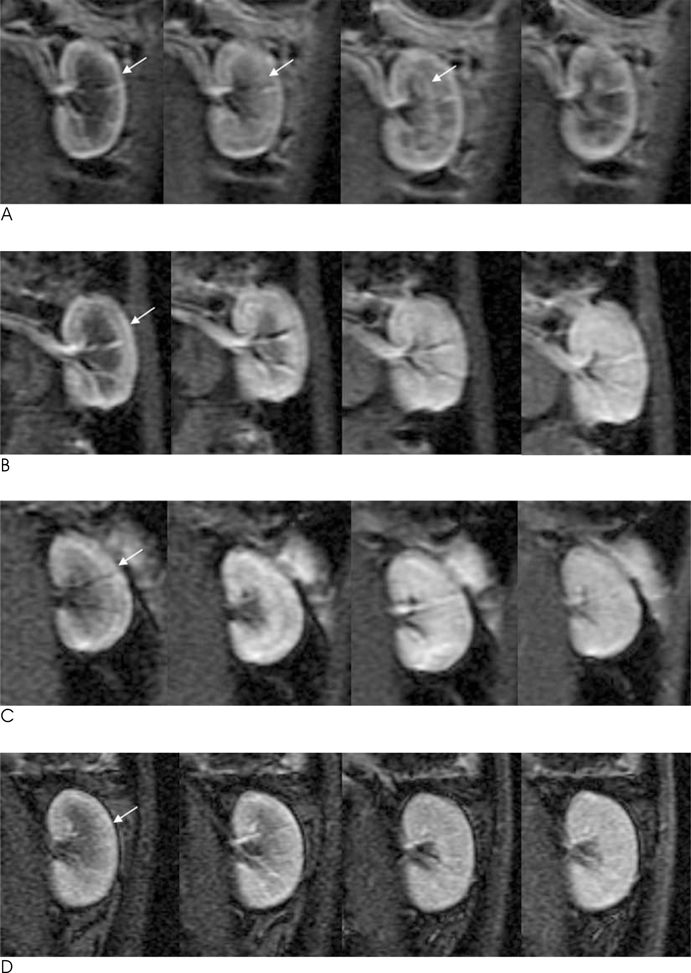
Twenty-four rabbits were randomly divided into four groups, including a sham operated group (n=3). Renal ischemia was induced for 30 minutes (group 1), 60 minutes (group 2) and 120 minutes (group 3). MR imaging was performed before ischemia as well as one hour, 24 hours, and 72 hours after reperfusion. A (99m)Tc-dimercaptosuccinic acid (DMSA) scintigraphy was performed before ischemia, as well as 24 hours and 72 hours after reperfusion. The signal-to-noise ratio (SNR) on the T2WI, time-relative signal intensity (%RSI) curve on dynamic enhanced images, and relative left renal uptake (%) on DMSA scan were obtained and compared to the histologic findings.
RESULTS
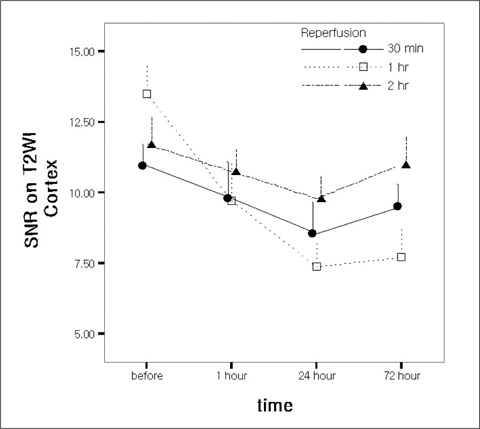
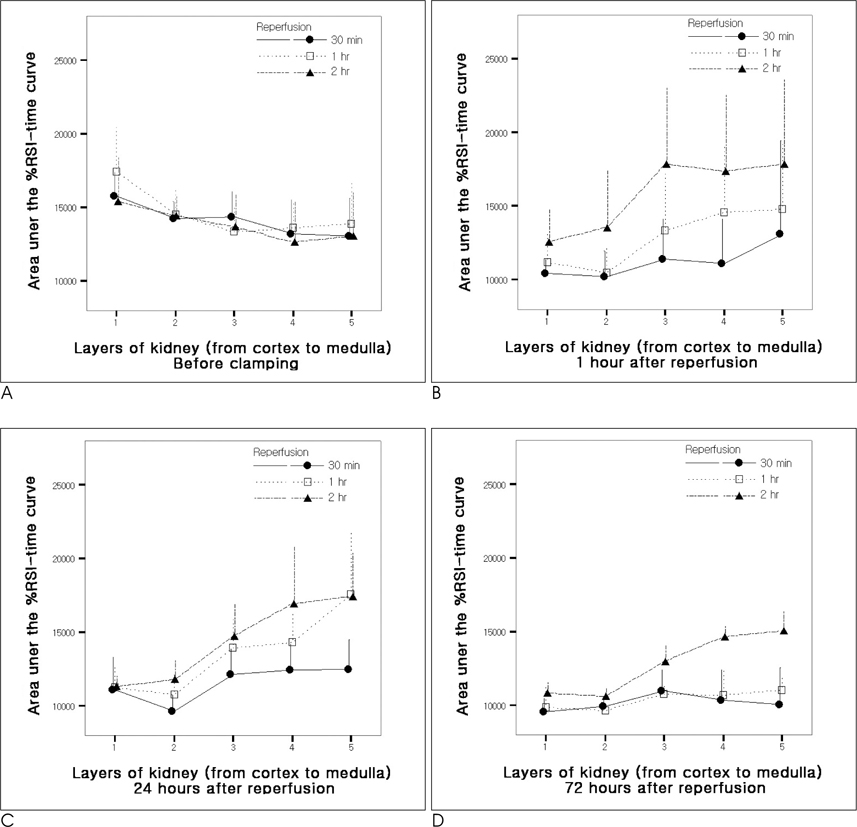
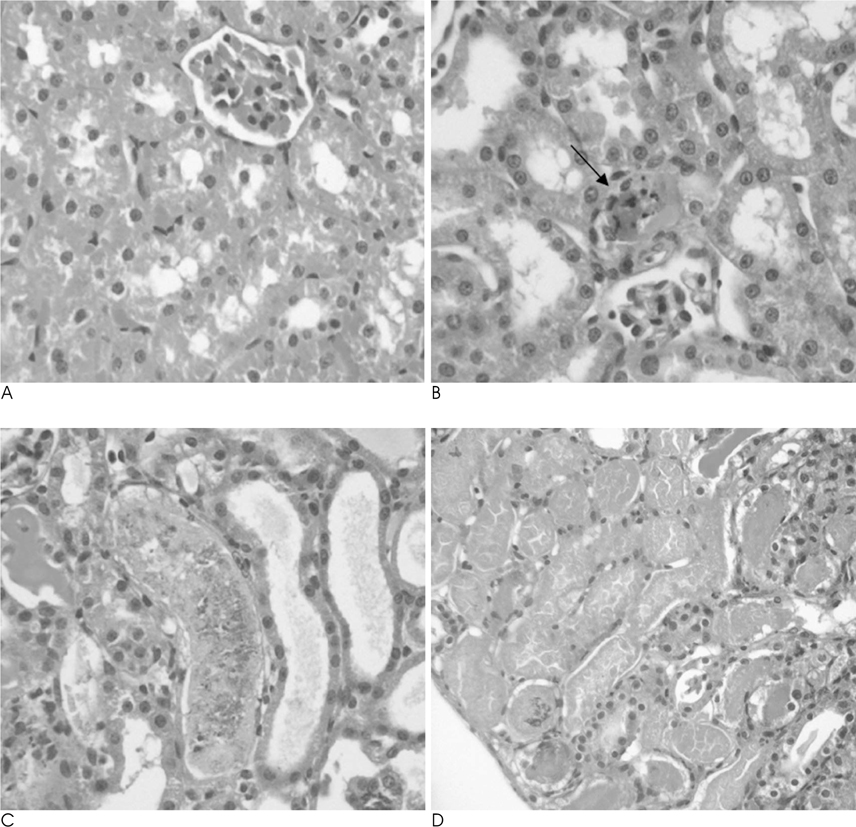
The SNR of the cortex on the T2WI changed significantly over the course of the reperfusion time (p<0.001), but was not significantly different among the ischemia groups. The area under the time-%RSI curve gradually decreased from cortex to inner medulla before ischemia, which was reversed and gradually increased after reperfusion. The areas under the time-%RSI curve of outer and inner medulla were significantly different among the ischemia groups (p=0.04, p=0.008). The relative renal uptake (%) of left kidney decreased significantly over the reperfusion time (p=0.03), and was also significantly different among the ischemia groups (p=0.005). Tubular cell necrosis was observed in 16 rabbits (76.2%). The histologic grades of group 3 were higher than those of group 1 and group 2 (p=0.002). Even in rabbits without tubular cell necrosis, the areas under the time-%RSI curves of the cortex, outer, and inner medulla after a 72 hour reperfusion time were significantly lower than those before ischemia (p=0.007, p=0.005, p=0.004).
CONCLUSION
The results of this study suggest that dynamic enhanced MR imaging could be a useful tool for the evaluation of renal ischemia and reperfusion injury.
MeSH Terms
Figure
Reference
-
1. Lechevallier E, Dussol B, Luccioni A, Thirion X, Vacher-Copomat H, Jaber K, et al. Posttransplantation acute tubular necrosis: risk factors and implications for graft survival. Am J Kidney Dis. 1998; 32:984–991.2. Daemen MA, Vreis BD, Buurman WA. Apoptosis and inflammation in renal reperfusion injury. Transplantation. 2002; 73:1693–1700.3. Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004; 66:480–485.4. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001; 281:F887–F899.5. Carvlin MJ, Arger PH, Kundel HL, Axel L, Dougherty L, Kassab EA, et al. Use of Gd-DTPA and fast gradient-echo and spin-echo MR imaging to demonstrate renal function in the rabbit. Radiology. 1989; 170:705–711.6. Prasad PV, Priatna A. Functional imaging of the kidneys with fast MRI techniques. Eur J Radiol. 1999; 29:133–148.7. Keogan MT, Edelman RR. Technologic advances in abdominal MR imaging. Radiology. 2001; 220:310–320.8. Laissy JP, Faraggi M, Lebtahi R, Soyer P, Brillet G, Mery JP, et al. Functional evaluation of normal and ischemic kidney by means of gadolinium-DOTA enhanced turboFLASH MR imaging: a preliminary comparison with 99mTc-MAG3 dynamic scintigraphy. Magn Reson Imaging. 1994; 12:413–419.9. Liu AS, Xie JX. Functional evaluation of normothermic ischemia and reperfusion injury in dog kidney by combining MR diffusionweighted imaging and Gd-DTPA enhanced first-pass perfusion. J Magn Reson Imaging. 2003; 17:683–693.10. Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983; 35:198–204.11. Choyke PL, Frank JA, Girton ME, Inscoe SW, Carvlin MJ, Black JL, et al. Dynamic Gd-DTPA-enhanced MR imaging of the kidney: experimental results. Radiology. 1989; 170:713–720.12. Suga K, Ogasawara N, Okazaki H, Sasai K, Matsunaga N. Functional assessment of canine kidneys after acute vascular occlusion on Gd-DTPA-enhanced dynamic echo-planar MR imaging. Invest Radiol. 2001; 36:659–676.13. Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol Renal Physiol. 2002; 282:F1140–F1149.14. Müller-Suur R, Gutsche HU. Tubular reabsorption of technetium-99m-DMSA. J Nucl Med. 1995; 36:1654–1658.15. Royal HD. Genitourinary system. In : Bernier DR, Christian P, Langan JK, editors. Nuclear Medicine: technology and techniques. 4th ed. St Louis: Mosby;1997. p. 389–406.16. Toosy N, McMorris EL, Grace PA, Mathie RT. Ischaemic preconditioning protects the rat kidney from reperfusion injury. BJU Int. 1999; 84:489–494.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitative evaluation of renal parenchymal perfusion using contrast-enhanced ultrasonography in renal ischemia-reperfusion injury in dogs
- Dynamic Contrast-Enhanced T2*-Weighted Imaging in Acute Cerebral Infarction: Usefulness in Assessment of Cerebral Hemodynamics
- Imaging Features of Gray-Scale and Contrast-Enhanced Color Doppler US for the Differentiation of Transient Renal Arterial Ischemia and Arterial Infarction
- Comparison of Gadolinium Polylysine and Gadopentetate in Contrast Enhanced MR Imaging of IVlyocardial Ischemia-Reperfusion in Cats
- MR Finding of Primary Renal Lymphoma: A Case Report