J Korean Soc Magn Reson Med.
2014 Dec;18(4):290-302. 10.13104/jksmrm.2014.18.4.290.
Clinical Applications of Neuroimaging with Susceptibility Weighted Imaging: Review Article
- Affiliations
-
- 1Department of Radiology, Seoul Veterans Hospital, Seoul, Korea. knroo@hanmail.net
- KMID: 2206926
- DOI: http://doi.org/10.13104/jksmrm.2014.18.4.290
Abstract
- PURPOSE
Susceptibility-weighted magnetic resonance (MR) sequence is three-dimensional (3D), spoiled gradient-echo pulse sequences that provide a high sensitivity for the detection of blood degradation products, calcifications, and iron deposits. This pictorial review is aimed at illustrating and discussing its main clinical applications.
MATERIALS AND METHODS
SWI is based on high-resolution, 3D, fully velocity-compensated gradient-echo sequences using both magnitude and phase images. To enhance the visibility of the venous structures, the magnitude images are multiplied with a phase mask generated from the filtered phase data, which are displayed at best after post-processing of the 3D dataset with the minimal intensity projection algorithm. A total of 200 patients underwent MR examinations that included SWI on a 3 tesla MR imager were enrolled.
RESULTS
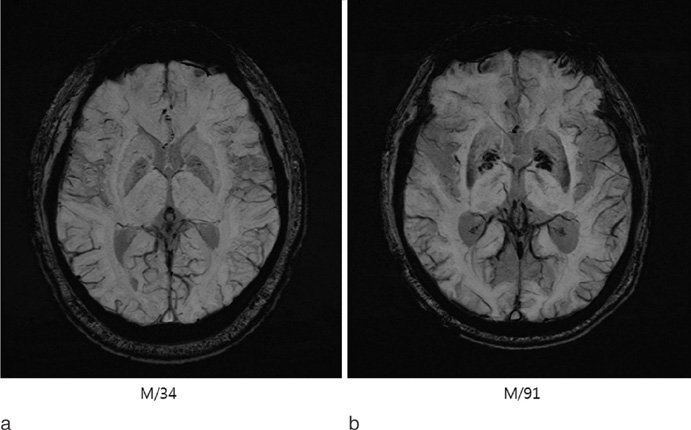
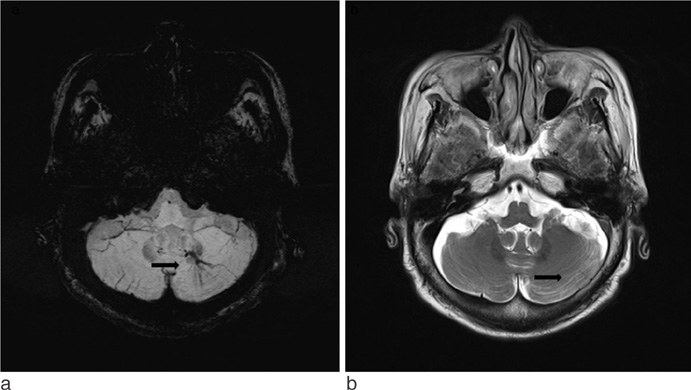
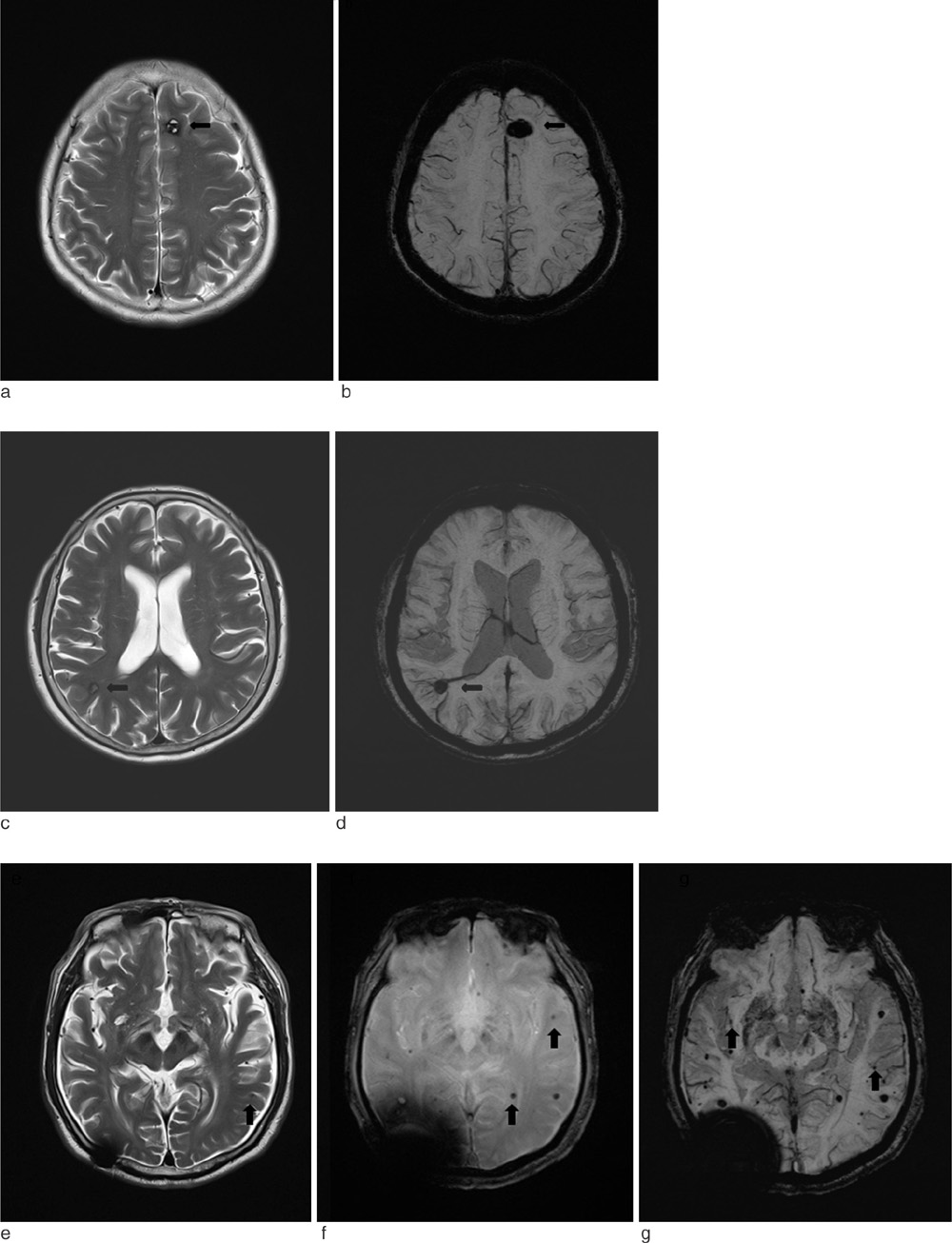
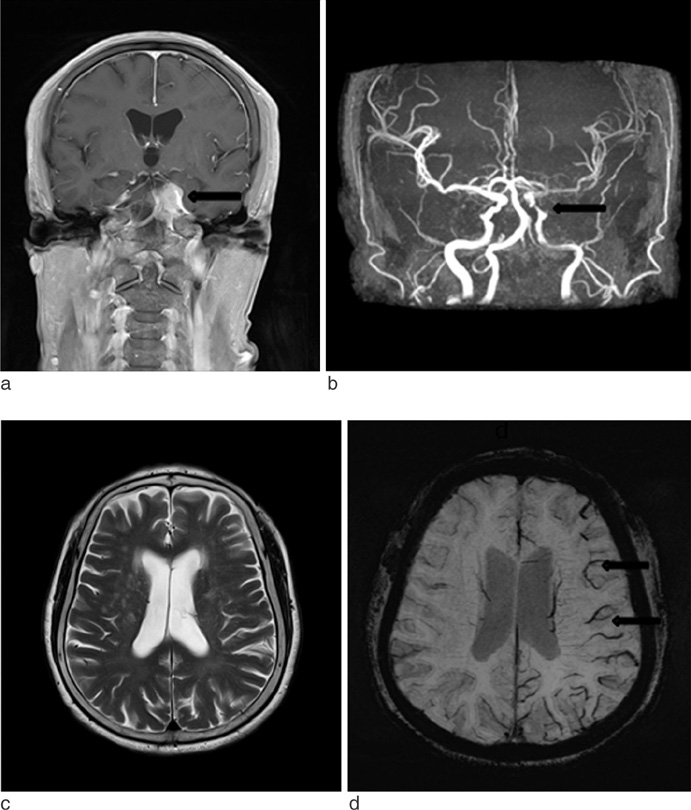
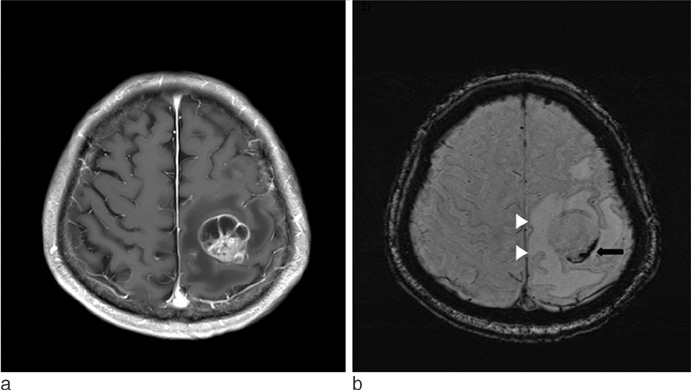
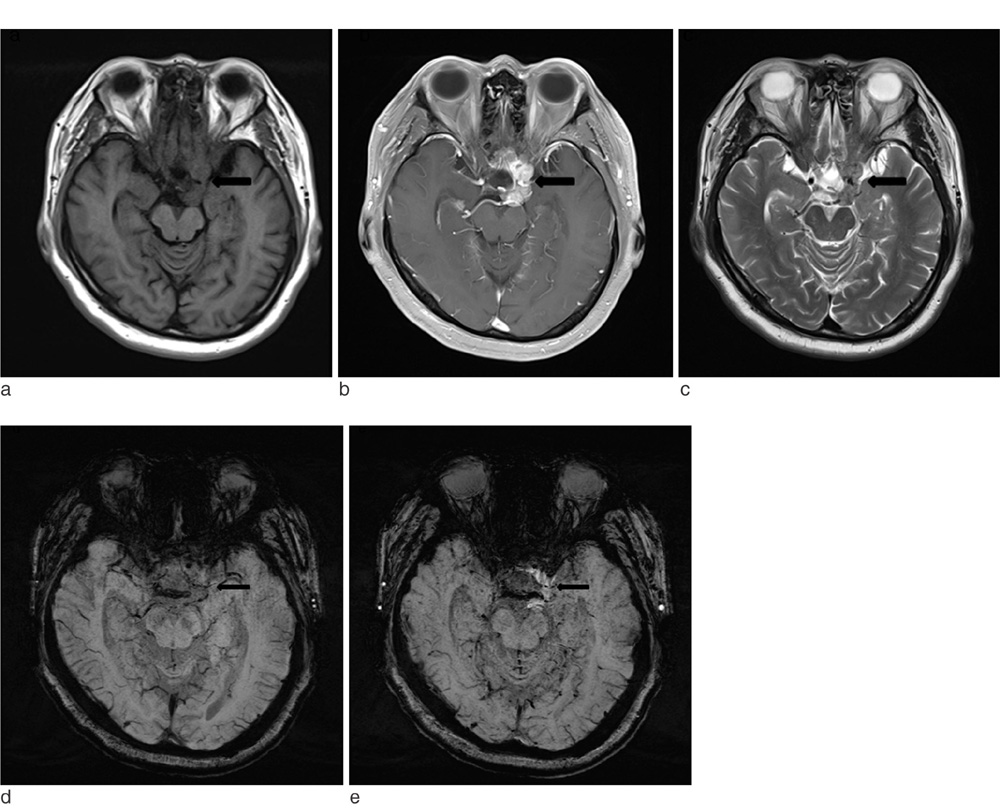
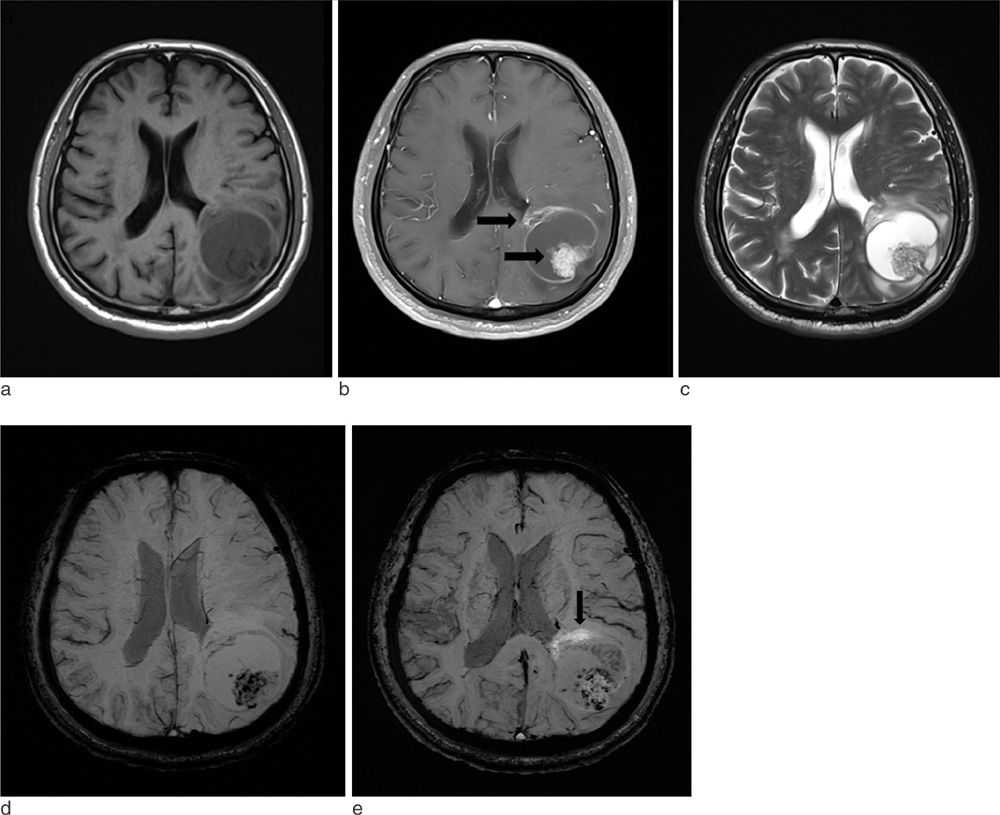
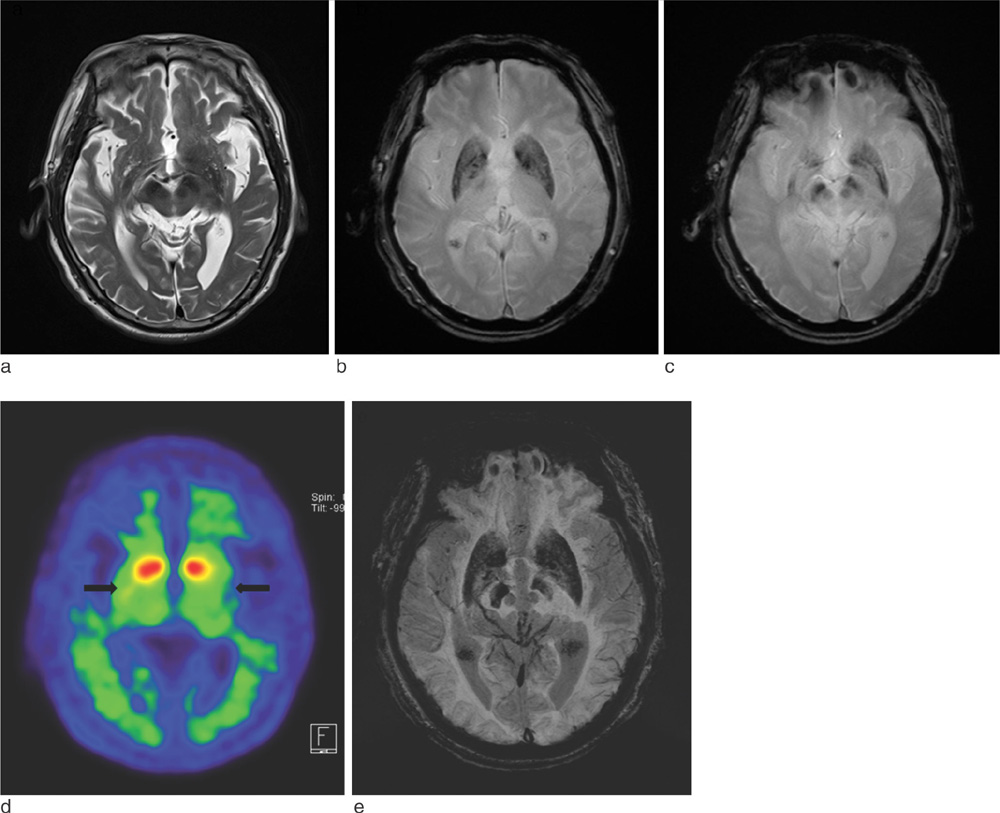
SWI is very useful in detecting multiple brain disorders. Among the 200 patients, 80 showed developmental venous anomaly, 22 showed cavernous malformation, 12 showed calcifications in various conditions, 21 showed cerebrovascular accident with susceptibility vessel sign or microbleeds, 52 showed brain tumors, 2 showed diffuse axonal injury, 3 showed arteriovenous malformation, 5 showed dural arteriovenous fistula, 1 showed moyamoya disease, and 2 showed Parkinson's disease.
CONCLUSION
SWI is useful in detecting occult low flow vascular lesions, calcification and microbleed and characterising diverse brain disorders.
MeSH Terms
Figure
Reference
-
1. Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997; 204:272–277.2. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009; 30:19–30.3. Wang D, Li WB, Wei XE, Li YH, Dai YM. An investigation of age-related iron deposition using susceptibility weighted imaging. PLoS One. 2012; 7:e50706. doi: 10.1371/journal.pone.0050706 Epub 2012 30.4. Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility weighted imaging. J Magn Reson Imaging. 2005; 22:439–450.5. Sarwar M, McCormick W. Intracerebral venous angioma: case report and review. Arch Neurol. 1978; 35:323–325.6. Kaplan HA, Aronson SM, Browder EJ. Vascular malformations of the brain. An anatomical study. J Neurosurg. 1961; 18:630–635.7. Wu Z, Mittal S, Kish K, Yu Y, Hu J, Haacke EM. Identification of calcification with magnetic resonance imaging using susceptibility-weighted imaging: a case study. J Magn Reson Imaging. 2009; 29:177–182.8. Santhosh K, Kesavadas C, Thomas B, Gupta AK, Thamburaj K, Kapilamoorthy TR. Susceptibility weighted imaging: a new tool in magnetic resonance imaging of stroke. Clin Radiol. 2009; 64:74–83.9. Radbruch A, Graf M, Kramp L, et al. Differentiation of brain metastases by percentagewise quantification of intratumoral-susceptibility-signals at 3Tesla. Eur J Radiol. 2012; 81:4064–4068. doi: 10.1016/j.ejrad.2012.06.016 Epub 2012 12.10. Sehgal V, Delproposto Z, Haddar D, et al. Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging. 2006; 24:41–51.11. Horie N, Morikawa M, Nozaki A, Hayashi K, Suyama K, Nagata I. "Brush Sign" on susceptibility-weighted MR imaging indicates the severity of moyamoya disease. AJNR Am J Neuroradiol. 2011; 32:1697–1702. doi: 10.3174/ajnr.A2568 Epub 2011 28.12. Thomas B, Somasundaram S, Thamburaj K, et al. Clinical applications of susceptibility weighted MR imaging of the brain -a pictorial review. Neuroradiology. 2008; 50:105–116.13. Bartzokis G, Cummings JL, Markham CH, et al. MRI evaluation of brain iron in earlier- and later-onset Parkinson's disease and normal subjects. Magn Reson Imaging. 1999; 17:213–222.14. Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury:improved detection and initial results. Radiology. 2003; 227:332–339.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Modern Brain Tumor Imaging
- Structural MR Imaging in the Diagnosis of Alzheimer's Disease and Other Neurodegenerative Dementia: Current Imaging Approach and Future Perspectives
- Artificial Intelligence in Neuroimaging: Clinical Applications
- Hypointense Rim on Susceptibility-Weighted Imaging in a Patient with Progressive Multifocal Leukoencephalopathy
- Neuroimaging of Vascular Dementia