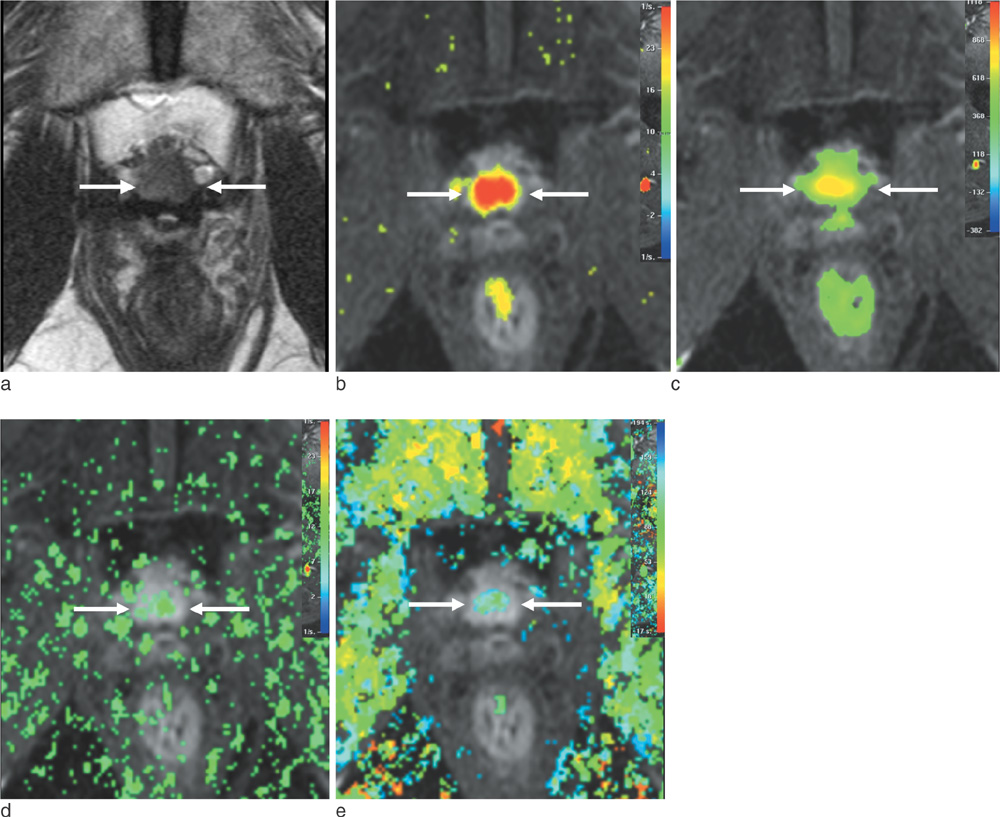
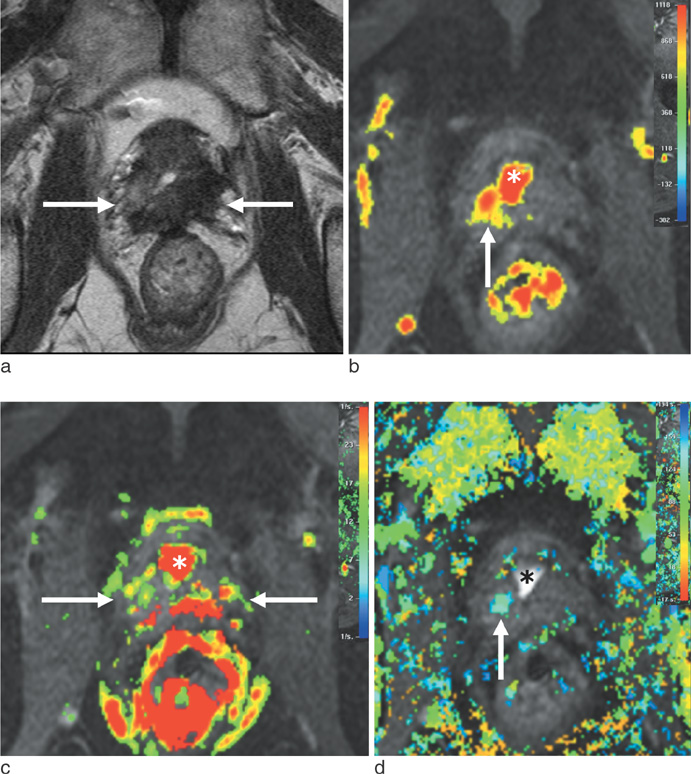
J Korean Soc Magn Reson Med.
2013 Sep;17(3):192-199. 10.13104/jksmrm.2013.17.3.192.
Dynamic Contrast-Enhanced MR Imaging in Detecting Local Tumor Progression after HIFU Ablation of Localized Prostate Cancer
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea. chankyokim@skku.edu
- 2Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea.
- KMID: 2206917
- DOI: http://doi.org/10.13104/jksmrm.2013.17.3.192
Abstract
- PURPOSE
To retrospectively evaluate the diagnostic performance of dynamic contrast-enhanced MR imaging (DCE-MRI) in detecting recurrent prostate cancer after HIFU of clinically localized cancer, as compared with T2-weighted imaging (T2WI).
MATERIALS AND METHODS
Twenty-six patients with increased prostate-specific antigen levels after HIFU were included in this study. All MR examinations were performed using T2WI and DCE-MRI, followed by transrectal ultrasound-guided biopsy. MRI and biopsy results were correlated in six prostate sectors. Residual or recurrent cancer after HIFU was defined as local tumor progression if biopsy results showed any cancer foci. Two independent readers interpreted the MR images.
RESULTS
Of 156 prostate sectors, 51 (33%) were positive for cancer in 17 patients. For detecting local tumor progression, the sensitivity of DCE-MRI and T2WI was 80% and 57% for reader 1 (P < 0.001) versus 84% and 61% for reader 2 (P < 0.001), respectively. The specificity and overall accuracy between DCE-MRI and T2WI showed no statistical difference in both readers (P > 0.05). Interobserver agreement of DCE-MRI and T2WI was moderate and fair, respectively.
CONCLUSION
For detecting local tumor progression of prostate cancer after HIFU, DCE-MRI was more sensitive than T2WI, with less interobserver variability.
Keyword
MeSH Terms
Figure
Reference
-
1. Aus G, Abbou CC, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2005; 48:546–551.2. Aus G. Current status of HIFU and cryotherapy in prostate cancer--a review. Eur Urol. 2006; 50:927–934. discussion 934.3. Poissonnier L, Chapelon JY, Rouviere O, et al. Control of prostate cancer by transrectal HIFU in 227 patients. Eur Urol. 2007; 51:381–387.4. Forsythe K, Blacksburg S, Stone N, Stock RG. Intensity-modulated radiotherapy causes fewer side effects than three-dimensional conformal radiotherapy when used in combination with brachytherapy for the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2012; 83:630–635.5. Orsi F, Arnone P, Chen W, Zhang L. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther. 2010; 6:414–420.6. Colombel M, Gelet A. Principles and results of high-intensity focused ultrasound for localized prostate cancer. Prostate Cancer Prostatic Dis. 2004; 7:289–294.7. Rebillard X, Gelet A, Davin JL, et al. Transrectal high-intensity focused ultrasound in the treatment of localized prostate cancer. J Endourol. 2005; 19:693–701.8. Gelet A, Chapelon JY, Poissonnier L, et al. Local recurrence of prostate cancer after external beam radiotherapy: early experience of salvage therapy using high-intensity focused ultrasonography. Urology. 2004; 63:625–629.9. Rouviere O, Lyonnet D, Raudrant A, et al. MRI appearance of prostate following transrectal HIFU ablation of localized cancer. Eur Urol. 2001; 40:265–274.10. Kirkham AP, Emberton M, Hoh IM, Illing RO, Freeman AA, Allen C. MR imaging of prostate after treatment with high-intensity focused ultrasound. Radiology. 2008; 246:833–844.11. Kim CK, Park BK, Lee HM, Kim SS, Kim E. MRI techniques for prediction of local tumor progression after high-intensity focused ultrasonic ablation of prostate cancer. AJR Am J Roentgenol. 2008; 190:1180–1186.12. Ben Cheikh A, Girouin N, Ryon-Taponnier P, et al. MR detection of local prostate cancer recurrence after transrectal high-intensity focused US treatment: preliminary results. J Radiol. 2008; 89:571–577.13. Punwani S, Emberton M, Walkden M, et al. Prostatic cancer surveillance following whole-gland high-intensity focused ultrasound: comparison of MRI and prostate-specific antigen for detection of residual or recurrent disease. Br J Radiol. 2012; 85:720–728.14. Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005; 235:728–739.15. Engelbrecht MR, Huisman HJ, Laheij RJ, et al. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology. 2003; 229:248–254.16. Futterer JJ, Engelbrecht MR, Huisman HJ, et al. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology. 2005; 237:541–549.17. Kim JK, Hong SS, Choi YJ, et al. Wash-in rate on the basis of dynamic contrast-enhanced MRI: usefulness for prostate cancer detection and localization. J Magn Reson Imaging. 2005; 22:639–646.18. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174.19. Donahue KM, Weisskoff RM, Parmelee DJ, et al. Dynamic Gd-DTPA enhanced MRI measurement of tissue cell volume fraction. Magn Reson Med. 1995; 34:423–432.20. Isebaert S, Van den Bergh L, Haustermans K, et al. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. J Magn Reson Imaging. 2013; 37:1392–1401.21. Ocak I, Bernardo M, Metzger G, et al. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: a study of pharmacokinetic parameters. AJR Am J Roentgenol. 2007; 189:849.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multidisciplinary Functional MR Imaging for Prostate Cancer
- A structured framework for optimizing high-intensity focused ultrasound ablative treatment in localized prostate cancer
- Medical imaging of prostate cancer
- Assessment of Local Tumor Progression After Image-Guided Thermal Ablation for Renal Cell Carcinoma
- Prostate Cancer: Added Value of Subtraction Dynamic Imaging in 3T Magnetic Resonance Imaging with a Phased-array Body Coil