Investig Clin Urol.
2019 Jul;60(4):312-318. 10.4111/icu.2019.60.4.312.
A structured framework for optimizing high-intensity focused ultrasound ablative treatment in localized prostate cancer
- Affiliations
-
- 1Department of Urology, IRCCS INRCA, Ancona, Italy. d.castellani@inrca.it
- 2Prostate Cancer Prevention Unit, IRCCS INRCA, Ancona, Italy.
- 3Department of Radiology, IRCCS INRCA, Ancona, Italy.
- KMID: 2450549
- DOI: http://doi.org/10.4111/icu.2019.60.4.312
Abstract
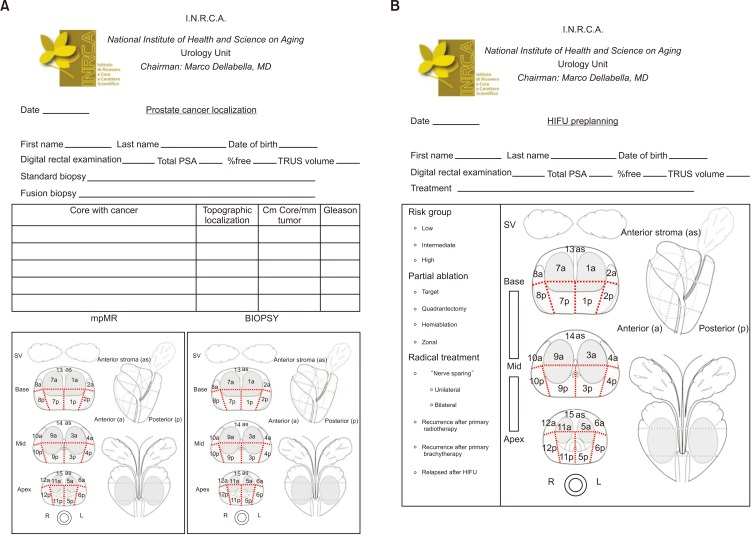
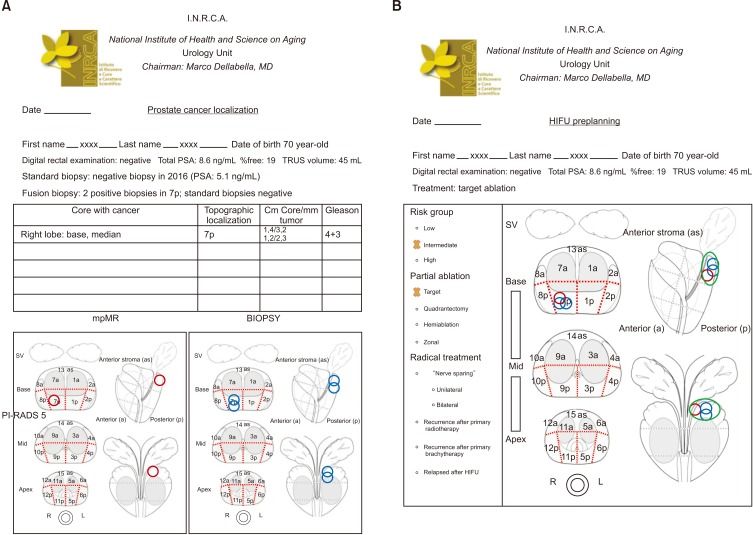
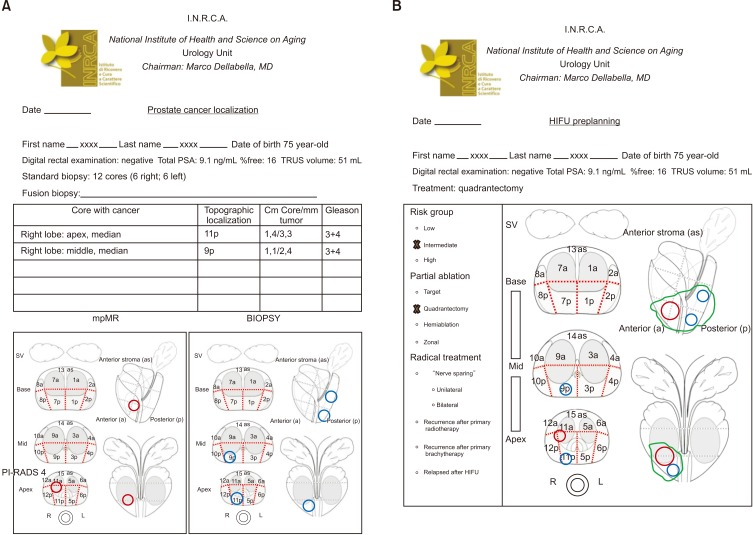
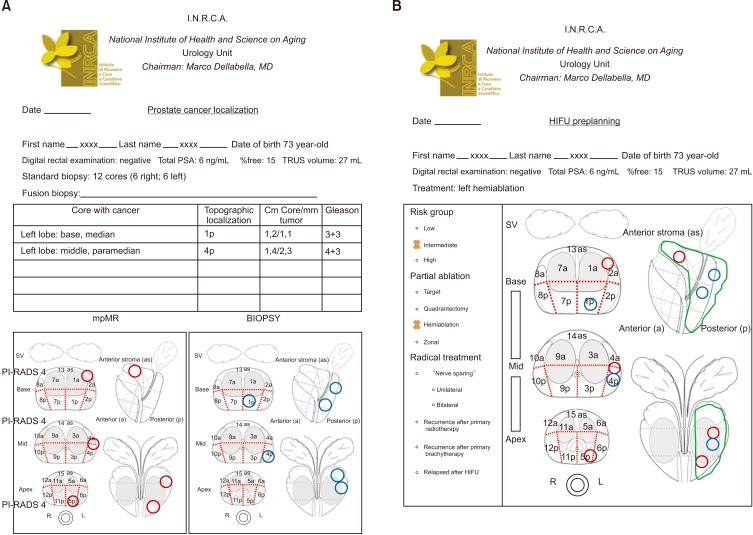
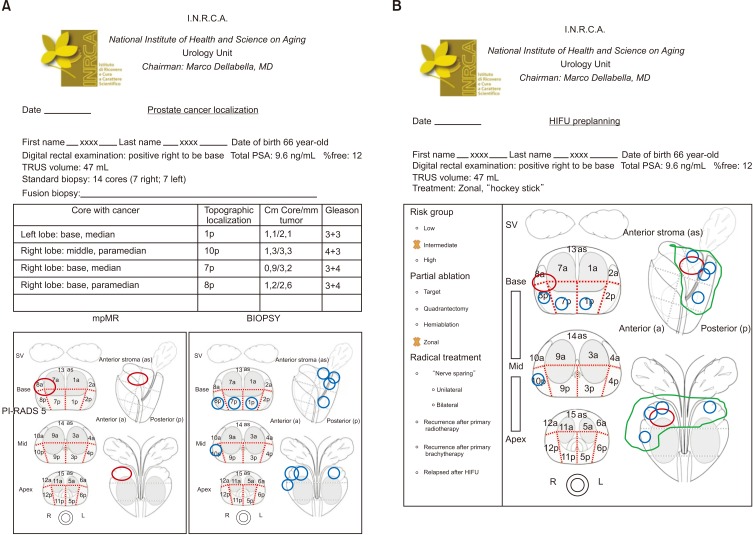
- High-intensity focused ultrasound (HIFU) treatment has recently been pursued to reduce radical treatment-related morbidity in low-to-intermediate-risk localized prostate cancer (PCa), especially in older men. The aim of this study was to develop a dedicated framework for HIFU therapy. All clinical data, such as risk categories, magnetic resonance with functional parametric imaging, and histopathology, are essential for driving proper HIFU treatment. All needed data can be added to the framework to localize areas that need to be treated. Once PCa areas have been featured, quantified, and located, planning can be adapted to drive accurate HIFU treatment. Our planning framework may be useful for all ablative therapies in order to standardize treatment for both clinical and scientific purposes.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017; 67:7–30. PMID: 28055103.
Article2. Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016; 375:1415–1424. PMID: 27626136.
Article3. Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016; 375:1425–1437. PMID: 27626365.
Article4. Valerio M, Cerantola Y, Eggener SE, Lepor H, Polascik TJ, Villers A, et al. New and established technology in focal ablation of the prostate: a systematic review. Eur Urol. 2017; 71:17–34. PMID: 27595377.
Article5. Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018; 74:422–429. PMID: 29960750.
Article6. Borycki EM, Kushniruk AW, Bellwood P, Brender J. Technology-induced errors. The current use of frameworks and models from the biomedical and life sciences literatures. Methods Inf Med. 2012; 51:95–103. PMID: 22101488.7. Woodrum DA, Kawashima A, Gorny KR, Mynderse LA. Prostate cancer: state of the art imaging and focal treatment. Clin Radiol. 2017; 72:665–679. PMID: 28385253.
Article8. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017; 71:618–629. PMID: 27568654.
Article9. Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011; 59:477–494. PMID: 21195536.
Article10. Sivaraman A, Barret E. Focal therapy for prostate cancer: an “À la Carte” approach. Eur Urol. 2016; 69:973–975. PMID: 26778462.
Article11. Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, et al. American Society of Clinical Oncology Statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015; 33:2563–2577. PMID: 26101248.
Article12. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) with NCCN Evidence Blocks [Internet]. cited 2019 Mar 30. Available from: https://www.nccn.org/evidenceblocks/default.aspx.13. Memorial Sloan Kettering Cancer Center Drug Pricing Lab. The Drug Abacus tool [Internet]. cited 2019 Mar 30. Available from: https://drugpricinglab.org/tools/drug-abacus/.14. Institute for Clinical and Economic Review. Overview of the ICER value assessment framework and update for 2017-2019 [Internet]. cited 2019 Mar 30. Available from: https://icer-review.org/wp-content/uploads/2017/06/ICER-value-assessment-framework-Updated-050818.pdf.15. Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 2015; 26:1547–1573. PMID: 26026162.
Article16. Slomiany M, Madhavan P, Kuehn M, Richardson S. Value frameworks in oncology: comparative analysis and implications to the pharmaceutical industry. Am Health Drug Benefits. 2017; 10:253–260. PMID: 28975009.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Focused ultrasound and prostate cancer
- Optimizing the Management of High-Risk, Localized Prostate Cancer
- Pain during Transrectal Ultrasound-Guided Prostate Biopsy and the Role of Periprostatic Nerve Block: What Radiologists Should Know
- Current status of high-intensity focused ultrasound for the management of uterine adenomyosis
- Treatment of Urethral/Bladder Neck Stricture After High-Intensity Focused Ultrasound for Prostate Cancer With Holmium: Yttrium-Aluminium-Garnet Laser