J Lung Cancer.
2011 Jun;10(1):49-55. 10.6058/jlc.2011.10.1.49.
Irinotecan and Cisplatin Combination Chemotherapy Plus Concurrent Thoracic Irradiation for Patients with Limited Disease Small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Gachon University Gil Hospital, Gachon University of Medicine and Science Graduate School of Medicine, Incheon, Korea. ekcho@gilhospital.com
- 2Department of Therapeutic Radiology and Oncology, Gachon University Gil Hospital, Gachon University of Medicine and Science Graduate School of Medicine, Incheon, Korea.
- 3Department of Internal Medicine, Samsung Seoul Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2199895
- DOI: http://doi.org/10.6058/jlc.2011.10.1.49
Abstract
- PURPOSE
To evaluate the antitumor activity and safety of irinotecan plus cisplatin combination chemotherapy with concurrent thoracic radiotherapy (TRT) in patients with limited disease (LD) small cell lung cancer (SCLC).
MATERIALS AND METHODS
Patients with pathologically-confirmed LD SCLC with the following inclusion criteria were retrospectively analyzed: age > or =18 years; measurable lesion; Eastern Cooperative Oncology Group Performance Status 0~2; chemotherapy naive; and adequate bone marrow and organ function. Patients received an intravenous (IV) infusion of irinotecan (35 mg/m2 on days 1, 8, and 15) and cisplatin (60 mg/m2 on day 1), which was repeated every 4 weeks for up to 6 cycles. Concurrent TRT was administered with the beginning of chemotherapy. Irinotecan was increased to 60 mg/m2 after completion of TRT. Patients with a complete response (CR) subsequently received prophylactic cranial irradiation.
RESULTS
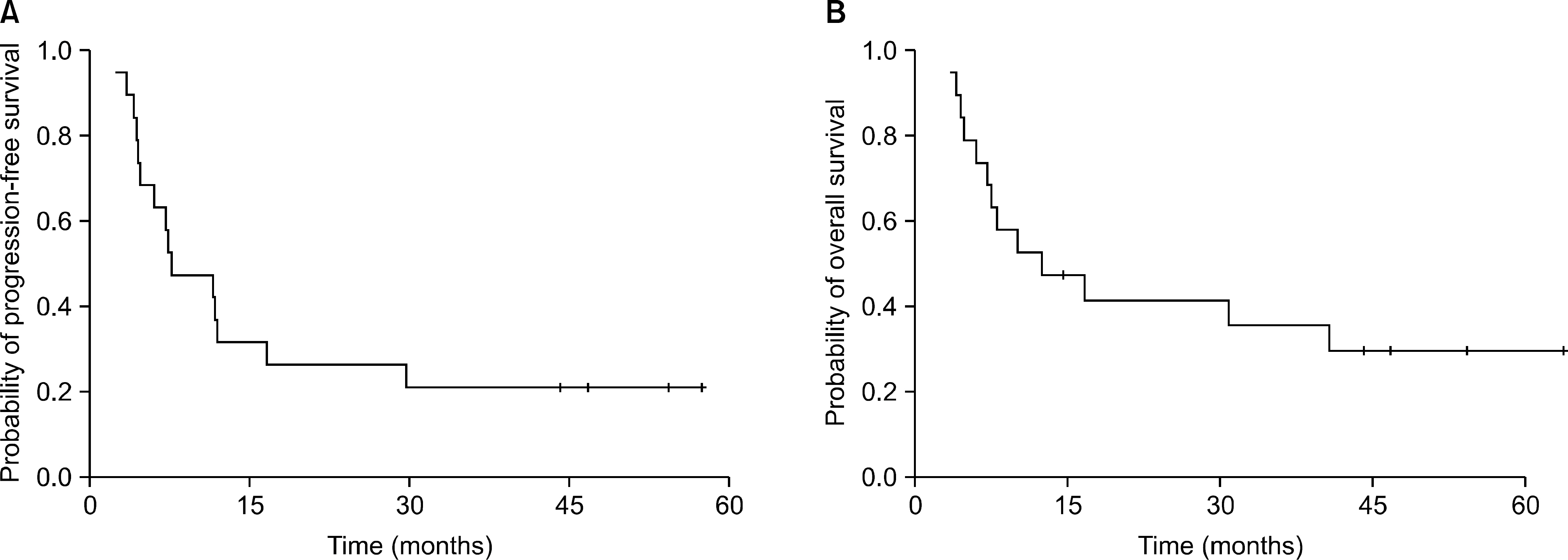
Nineteen patients were analyzed. There were 8 patients (42.1%) with CR, 9 patients (47.4%) with partial response, and 1 patient each (5.3%) with stable disease and progressive disease (PD). The overall response rate was 89.5%. The median progression-free survival was 7.6 months (95% confidence interval [CI], 1.3~14.0 months) and the median overall survival was 12.4 months (95% CI, 0.5~24.2 months). The 2-year survival rate of the CR patients was 75.0%. No grade 4 hematologic toxicity was reported. Frequently reported toxicities were nausea (10 patients), radiation-induced pneumonitis (10 patients), and neutropenia (6 patients). Radiation-related severe toxicities were frequently reported. Three patients had treatment-related deaths.
CONCLUSION
This study supports the activity and tolerability of irinotecan plus cisplatin with concurrent TRT in patients with LD SCLC.
Keyword
MeSH Terms
Figure
Reference
-
1. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epide-miologic, and end results database. J Clin Oncol. 2006; 24:4539–4544.
Article2. Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005; 366:1385–1396.
Article3. Cooper S, Spiro SG. Small cell lung cancer: treatment review. Respirology. 2006; 11:241–248.
Article4. Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol. 2008; 26:4261–4267.
Article5. Schmittel AH, Sebastian M, von Weikersthal LF, et al. Irinotecan plus carboplatin versus etoposide plus carboplatin in extensive disease small cell lung cancer: Results of the German randomized phase III trial. J Clin Oncol. 2009; 27((Suppl 15)):8029.
Article6. Kim YS, Park SH, Kyung SY, et al. Irinotecan plus carboplatin in patients with extensive-disease small-cell lung cancer. Med Oncol. 2011; 28:342–350.
Article7. Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002; 346:85–91.
Article8. Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006; 24:2038–2043.
Article9. Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharma-cogenomic results from SWOG S0124. J Clin Oncol. 2009; 27:2530–2535.
Article10. Simon M, Argiris A, Murren JR. Progress in the therapy of small cell lung cancer. Crit Rev Oncol Hematol. 2004; 49:119–133.
Article11. Huncharek M, McGarry R. A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer. Oncologist. 2004; 9:665–672.
Article12. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992; 10:890–895.
Article13. Yokoyama A, Kurita Y, Saijo N, et al. Dose-finding study of irinotecan and cisplatin plus concurrent radiotherapy for unresectable stage III non-small-cell lung cancer [seecom-ments]. Br J Cancer. 1998; 78:257–262.14. Takiguchi Y, Uruma R, Asaka-Amano Y, et al. Phase I study of cisplatin and irinotecan combined with concurrent hyperfractionated accelerated thoracic radiotherapy for locally advanced non-small cell lung carcinoma. Int J Clin Oncol. 2005; 10:418–424.
Article15. Oka M, Fukuda M, Kuba M, et al. Phase I study of irinotecan and cisplatin with concurrent split-course radiotherapy in limited-disease small-cell lung cancer. Eur J Cancer. 2002; 38:1998–2004.
Article16. Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst. 1991; 83:855–861.
Article17. Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992; 10:282–291.
Article18. Han JY, Cho KH, Lee DH, et al. Phase II study of irinotecan plus cisplatin induction followed by concurrent twice-daily thoracic irradiation with etoposide plus cisplatin chemotherapy for limited-disease small-cell lung cancer. J Clin Oncol. 2005; 23:3488–3494.
Article19. Kubota K, Nishiwaki Y, Sugiura T, et al. Pilot study of concurrent etoposide and cisplatin plus accelerated hyperfractionated thoracic radiotherapy followed by irinotecan and cisplatin for limited-stage small cell lung cancer: Japan Clinical Oncology Group 9903. Clin Cancer Res. 2005; 11:5534–5538.
Article20. Saito H, Takada Y, Ichinose Y, et al. Phase II study of etoposide and cisplatin with concurrent twice-daily thoracic radiotherapy followed by irinotecan and cisplatin in patients with limited-disease small-cell lung cancer: West Japan Thoracic Oncology Group 9902. J Clin Oncol. 2006; 24:5247–5252.
Article21. Jeong HC, Lee SY, Lee SY, et al. Phase II study of irinotecan plus cisplatin with concurrent radiotherapy for the patients with limited-disease small-cell lung cancer. Lung Cancer. 2006; 53:361–366.
Article22. Sohn JH, Moon YW, Lee CG, et al. Phase II trial of irinotecan and cisplatin with early concurrent radiotherapy in limited-disease small-cell lung cancer. Cancer. 2007; 109:1845–1950.
Article23. Quoix E, Purohit A, Faller-Beau M, Moreau L, Oster JP, Pauli G. Comparative prognostic value of lactate dehydrogenase and neuron-specific enolase in small-cell lung cancer patients treated with platinum-based chemotherapy. Lung Cancer. 2000; 30:127–134.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Small Cell Lung Cancer
- Treatment of Small Cell Lung Cancer
- Phase II Study of Induction Irinotecan + Cisplatin Chemotherapy Followed by Concurrent Irinotecan + Cisplatin Plus Twice-Daily Thoracic Radiotherapy
- Phase II Trial of Irinotecan plus Cisplatin Combination as First Line Therapy for Patients with Small cell Lung Cancer
- High-Dose Involved Field Radiotherapy and Concurrent Chemotherapy for Limited-Disease Small Cell Lung Cancer