J Lung Cancer.
2010 Dec;9(2):85-90. 10.6058/jlc.2010.9.2.85.
High-Dose Involved Field Radiotherapy and Concurrent Chemotherapy for Limited-Disease Small Cell Lung Cancer
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. jskim@snubh.org
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 3Department of Radiation Oncology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2199911
- DOI: http://doi.org/10.6058/jlc.2010.9.2.85
Abstract
- PURPOSE
We wanted to evaluate the effect of high dose involved field radiotherapy and concurrent chemotherapy for treating patients with limited disease, small cell lung cancer.
MATERIALS AND METHODS
We reviewed the medical records of 37 patients who had a limited stage of small cell lung cancer. All the patients were treated with induction chemotherapy followed by definitive radiotherapy and concurrent chemotherapy. The radiation dose was 60 Gy for 31 patients and 50~58 Gy for 6 patients with once-daily 2 Gy fractions. Elective nodal irradiation was not performed. The chemotherapy regimen was either combinations of etoposide and cisplatin or irinotecan and cisplatin. Prophylactic cranial irradiation of 25 Gy at 2.5 Gy per fraction was administered to the patients who had a complete or near complete response. The median follow-up period was 17 months (range, 5~57).
RESULTS
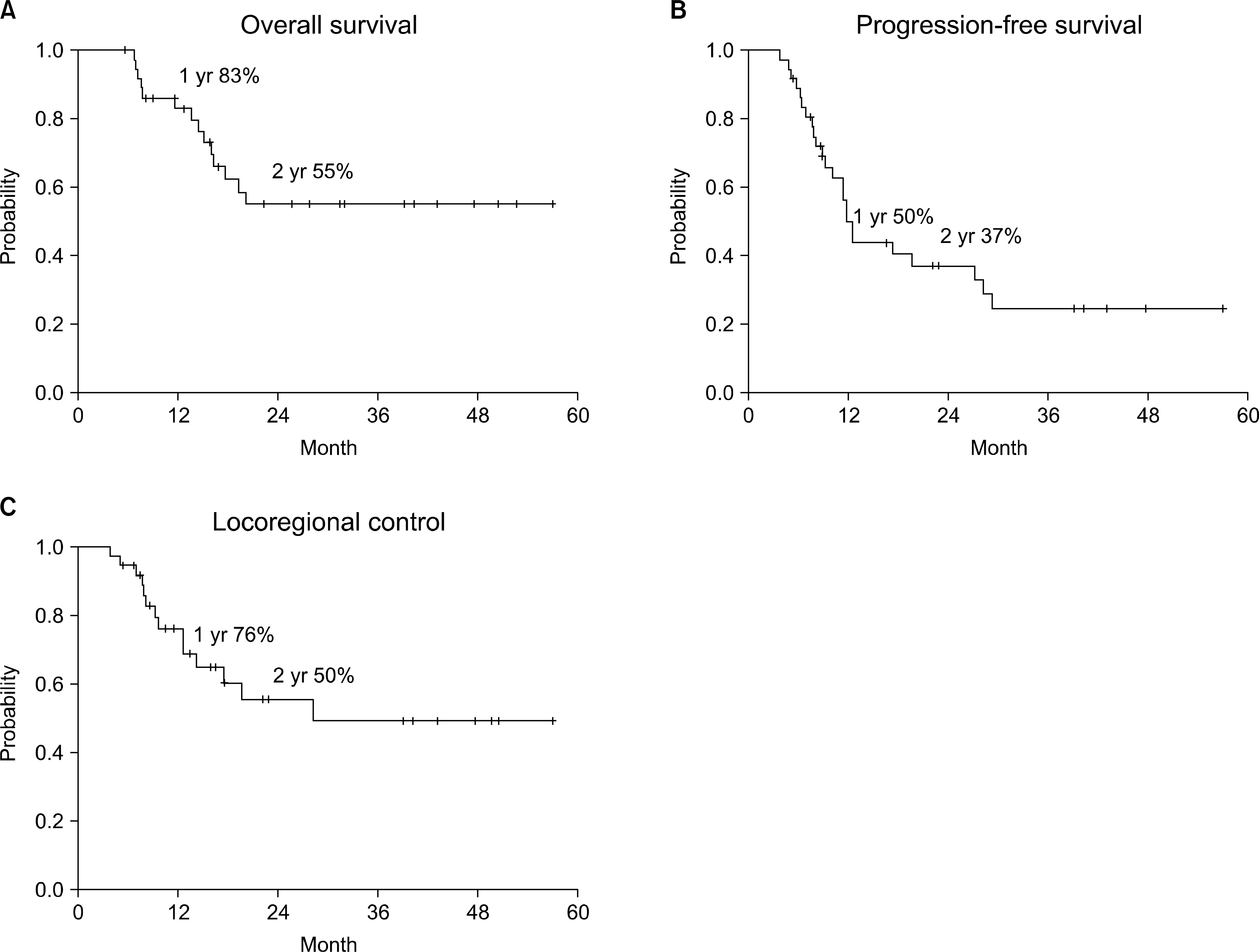
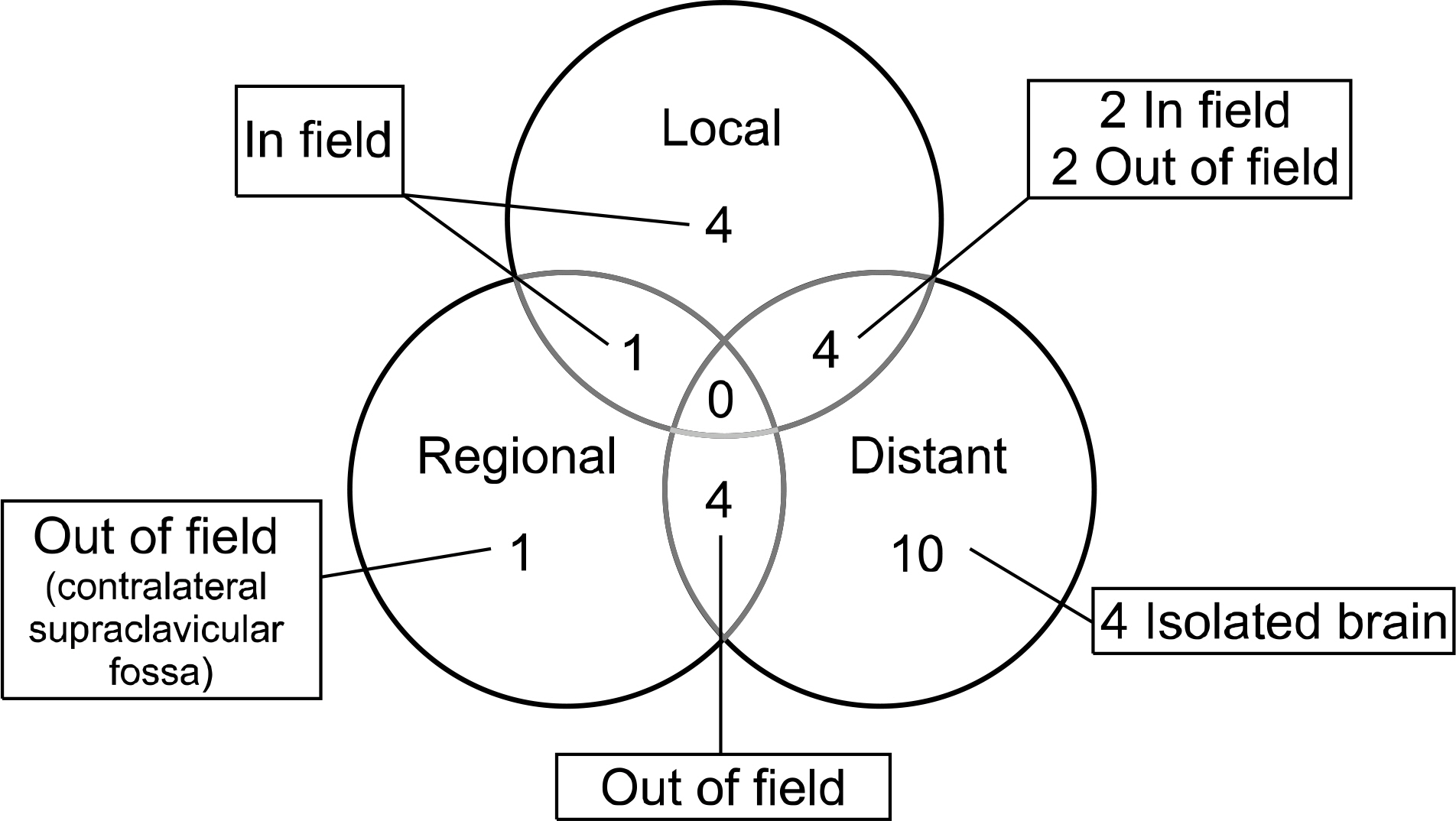
The 2-year overall survival and locoregional control rates were both 55%. A complete response was achieved in 17 patients (46%), a partial response was achieved in 19 patients (51%) and 1 patient (3%) had progressive disease. Seven patients experienced tumor recurrence in the radiation field and four of those recurrences were isolated local recurrences. There was only one isolated regional recurrence outside the radiation field. Grade 3 treatment-related esophageal toxicity occurred in 2 patients. Two patients died of treatment-related pulmonary complications.
CONCLUSION
Involved field radiotherapy of 60 Gy can achieve favorable survival and a low rate of isolated nodal failure outside the radiation field. However, a considerable number of patients still experienced in-field failure. Further studies to establish the optimal radiation doses and fractionation are needed in the future.
MeSH Terms
Figure
Reference
-
References
1. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006; 24:4539–4544.
Article2. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A metaanalysis. J Clin Oncol. 1992; 10:890–895.
Article3. Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002; 20:3054–3060.
Article4. Pignon JP, Arriagada R, Ihde DC, et al. A metaanalysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992; 327:1618–1624.
Article5. Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993; 11:336–344.
Article6. Bogart JA. Rationale for phase III trials of thoracic radiation therapy doses in limited-stage small-cell lung cancer. Clin Lung Cancer. 2008; 9:202–205.
Article7. Kubota K, Nishiwaki Y, Sugiura T, et al. Pilot study of concurrent etoposide and cisplatin plus accelerated hyperfractionated thoracic radiotherapy followed by irinotecan and cisplatin for limited-stage small cell lung cancer: Japan Clinical Oncology Group 9903. Clin Cancer Res. 2005; 11:5534–5538.
Article8. Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002; 346:85–91.
Article9. Sohn JH, Moon YW, Lee CG, et al. Phase II trial of irinotecan and cisplatin with early concurrent radiotherapy in limited-disease small-cell lung cancer. Cancer. 2007; 109:1845–1950.
Article10. Han JY, Cho KH, Lee DH, et al. Phase II study of irinotecan plus cisplatin induction followed by concurrent twice-daily thoracic irradiation with etoposide plus cisplatin chemotherapy for limited-disease small-cell lung cancer. J Clin Oncol. 2005; 23:3488–3494.
Article11. Xenidis N, Kotsakis A, Kalykaki A, et al. Etoposide plus cisplatin followed by concurrent chemoradiotherapy and irinotecan plus cisplatin for patients with limited-stage small cell lung cancer: a multicenter phase II study. Lung Cancer. 2010; 68:450–454.
Article12. Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999; 340:265–271.
Article13. Choi NC, Herndon J, Rosenman J, et al. Long term survival data from CALGB 8837: radiation dose escalation and concurrent chemotherapy (CT) in limited stage small cell lung cancer (LD-SCLC). Possible radiation dose-survival relationship. Proc Am Soc Clin Oncol. 2002; 21:298a. (abstr 1190).14. Bogart JA, Herndon JE 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004; 59:460–468.
Article15. Choi NC, Herndon JE 2nd, Rosenman J, et al. Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol. 1998; 16:3528–3536.
Article16. Tomita N, Kodaira T, Hida T, et al. The impact of radiation dose and fractionation on outcomes for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2010; 76:1121–1126.
Article17. Choi NC, Carey RW. Importance of radiation dose in achieving improved locoregional tumor control in limited stage small-cell lung carcinoma: an update. Int J Radiat Oncol Biol Phys. 1989; 17:307–310.
Article18. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216.19. World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization;1979.20. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003; 13:176–181.
Article21. Edge SB. AJCC cancer staging manual. 7th ed.New York: Springer;2009.22. Roof KS, Fidias P, Lynch TJ, et al. Radiation dose escalation in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003; 57:701–708.
Article23. De Ruysscher D, Bremer RH, Koppe F, et al. Omission of elective node irradiation on basis of CT-scans in patients with limited disease small cell lung cancer: a phase II trial. Radiother Oncol. 2006; 80:307–312.
Article24. van Loon J, De Ruysscher D, Wanders R, et al. Selective nodal irradiation on basis of (18)FDG-PET scans in limited-disease small-cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2010; 77:329–336.25. Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J, et al. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and metaanalysis of randomised controlled trials. Cancer Treat Rev. 2007; 33:461–473.
Article26. De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006; 24:1057–1063.
Article27. Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004; 59:943–951.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Locally Advanced Non-small Cell Lung Cancer
- Chemotherapy for Small Cell Lung Cancer
- Thoracic Radiotherapy Combined with Chemotherapy in Patients with Limited-stage Small-cell Lung Cancer
- Investigating Effective Combinations of Anti-cancer Drugs and Radiation Therapy for Treating Non-small Cell Lung Cancer with Using Two Cell Lines
- Concurrent Hyperfractionated Radiotherapy with CDDP / VP-16 Chemotherapy in Limited Stage Small Cell Lung Cancer