J Korean Med Assoc.
2012 Mar;55(3):279-291. 10.5124/jkma.2012.55.3.279.
Systematic review for new health technology assessment
- Affiliations
-
- 1Center for New Health Technology Assessment, National Evidence-based Healthcare Collaborating Agency, Seoul, Korea. sunarea68@neca.re.kr
- KMID: 2192718
- DOI: http://doi.org/10.5124/jkma.2012.55.3.279
Abstract
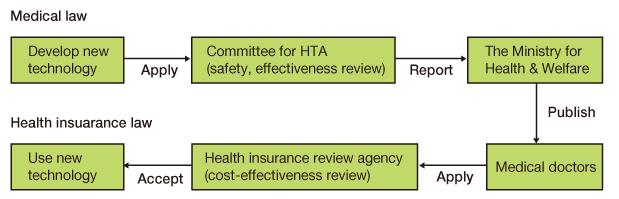
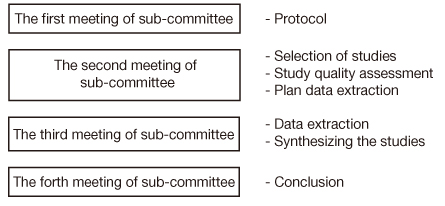
- We used a systematic review to evaluate the safety and effectiveness of a new health technology in Korea. The results of the systematic review are being used by the Health Insurance Review and Assessment Service when the Ministry of Health and Welfare introduces new medical technology. The purpose of this study is to introduce this systematic review, which is the main methodology for evaluating the safety and effectiveness of new medical technology, and to share our experience of performing a systematic review to guide the reader in performing a systematic literature review accurately and easily. This paper presents the process of new health technology evaluation using a systematic review. A systematic review involves collecting current available evidence of health technology systematically. According to the evaluation process, in the first meeting of sub-committee, we develop a systematic review protocol including PICO and criteria for inclusion/exclusion. In the second meeting of sub-committee, we search comprehensively for appropriate literature according to the clinical question and to select in a clear and reproducible method. We also assess study quality, considering the internal validity and external validity of the selected literature, and make a table of extracted data. In the third meeting of sub-committee, we extract general information, study characteristics information, and study outcome information, and synthesize the outcomes. In the forth meeting of sub-committee, we finalize the conclusions based on synthesizing the studies. After the subcommittees' assessments, the results are presented to the Committee for New Health Technology. Finally, we report this result to the Ministry of Health and Welfare. The systematic review is useful for helping policymakers make decisions about the introduction of new health technology based on evidence. It enables people to minimize confusion due to weak evidence for health technology. In case of domestically developed technology and technology for rare disease, it is difficult assess health technology due to a lack of evidence, so assessing raw data (charts, results of clinical trials) is needed in addition to a systematic review. Furthermore, the government should support clinical studies to develop evidence on new health technologies with potential benefit, and the introduction of a conditional coverage decision in the new health technology assessment system is necessary to deal with uncertainty.
Figure
Reference
-
1. Municipal Rule on National Health Insurance Reimbursement: amended by ordinance of the Korean Ministry of Health and Welfare. No. 140. 2009. 11. 30.2. New Health Technologies Decision and Adjustment Standard Act: amended by act of the Korean Ministry of Health and Welfare. No. 2009-3. 2009. 08. 24.3. Lee SH, Sul AR, Jung YJ. Australian Health Technology Assessment Agency meeting report. 2011. Seoul: National Evidence-based Healthcare Collaboratory Agency.4. Municipal Rule on New Health Technology Municipal: partially amended by the ordinance of the Korean Ministry of Health and Welfare. No. 1. 2009. 03. 03.5. Lee SH. Lee SH, editor. New health technology assessment. Function and role of Health Insurance Review & Assessment Service. 2009. Seoul: Health Insurance Review & Assessment Service;495–507.6. University of York. NHS Centre for Reviews and Dissemination. Undertaking systematic reviews of research on effectiveness: CRD's guidance for those carrying out or commissioning reviews. 2001. 2nd ed. York: Centre for Reviews and Dissemination, University of York.7. Health Insurance Review & Assessment Service. Korean Ministry of Health and Welfare. Report of heath technology assessment demonstration agency operating. 2005. Seoul: Health Insurance Review & Assessment Service;378.8. Health Insurance Review & Assessment Service. Radiofrequency ablation of liver tumors. 2004. Seoul: Health Insurance Review & Assessment Service.9. Bidwell S, Jensen MF. Topfer LA, Auston I, editors. National Information Center on Health Services Research & Health Care Technology (US). Using a search protocol to identify sources of information: the COSI model. Etext on health technology assessment (HTA) information resources. 2004. Bethesda: US National Library of Medicine, National Institutes of Health, Health & Human Services.10. Notes on the use of methodology checklist 1: systematic reviews and meta-analyses [Internet]. Scottish Intercollegiate Guidelines Network. c2001-2011. cited 2012 Feb 27. Edinburgh: Scottish Intercollegiate Guidelines Network;Available from: http://www.sign.ac.uk/guidelines/fulltext/50/notes1.html.11. Dawes M, Davies P, Gray A, Mant J, Seers K, Snowball R. Evidence-based practice: a primer for health care professionals. 2005. 2nd ed. Edinburgh: Elsevier;277.12. PRISMA 2009 flow diagram [Internet]. cited 2012 Feb 27. [place unknown]: PRISMA;Available from: http://www.prisma-statement.org/2.1.4%20-%20PRISMA%20Flow%202009%20Diagram.pdf.13. Khan KS, Kunz R, Kleijnen J, Antes G. Royal Society of Medicine (Great Britain). Systematic reviews to support evidence-based medicine: how to review and apply findings of healthcare research. 2011. 2nd ed. London: Royal Society of Medicine;201.14. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [Internet]. 2011. Cambridge: The Cochrane Collaboration;Available from: http://www.cochrane-handbook.org.15. Guyatt G, Rennie D. Evidence-Based Medicine Working Group. American Medical Association. User's guides to the medical literature. 2005. Chicago: AMA Press;706.16. Lee SH, Jang SY, Jung JH. HER-2 gene silver in situ hybridization in gastric adenocarinoma. 2011. Seoul: Korean Ministry of Health and Welfare;67.17. Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001. 323:157–162.
Article18. Greenhalgh T. Papers that summarise other papers: systematic reviews and meta-analyses. BMJ. 1997. 315:672–675.
Article19. Chalmers I, Altman DG. Systematic reviews. 1995. London: BMJ Publishing.20. National Health and Medical Research Council. How to review the evidence: systematic identification and review of the scientific literature. 2000. Canberra: National Health and Medical Research Council.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evidence Base Medicine and Pre-Appraised Resources
- New Health Technology before nHTA is also Uninsured Benefit
- A Systematic Review of Birth Experience Assessment Instrument
- Value-Based Health Technology Assessment and Health Informatics
- The current status and future direction of Korean health technology assessment system