J Korean Med Assoc.
2014 Nov;57(11):906-911. 10.5124/jkma.2014.57.11.906.
The current status and future direction of Korean health technology assessment system
- Affiliations
-
- 1National Evidence-based Healthcare Collaborating Agency, Seoul, Korea. jahn@neca.re.kr
- KMID: 1958390
- DOI: http://doi.org/10.5124/jkma.2014.57.11.906
Abstract
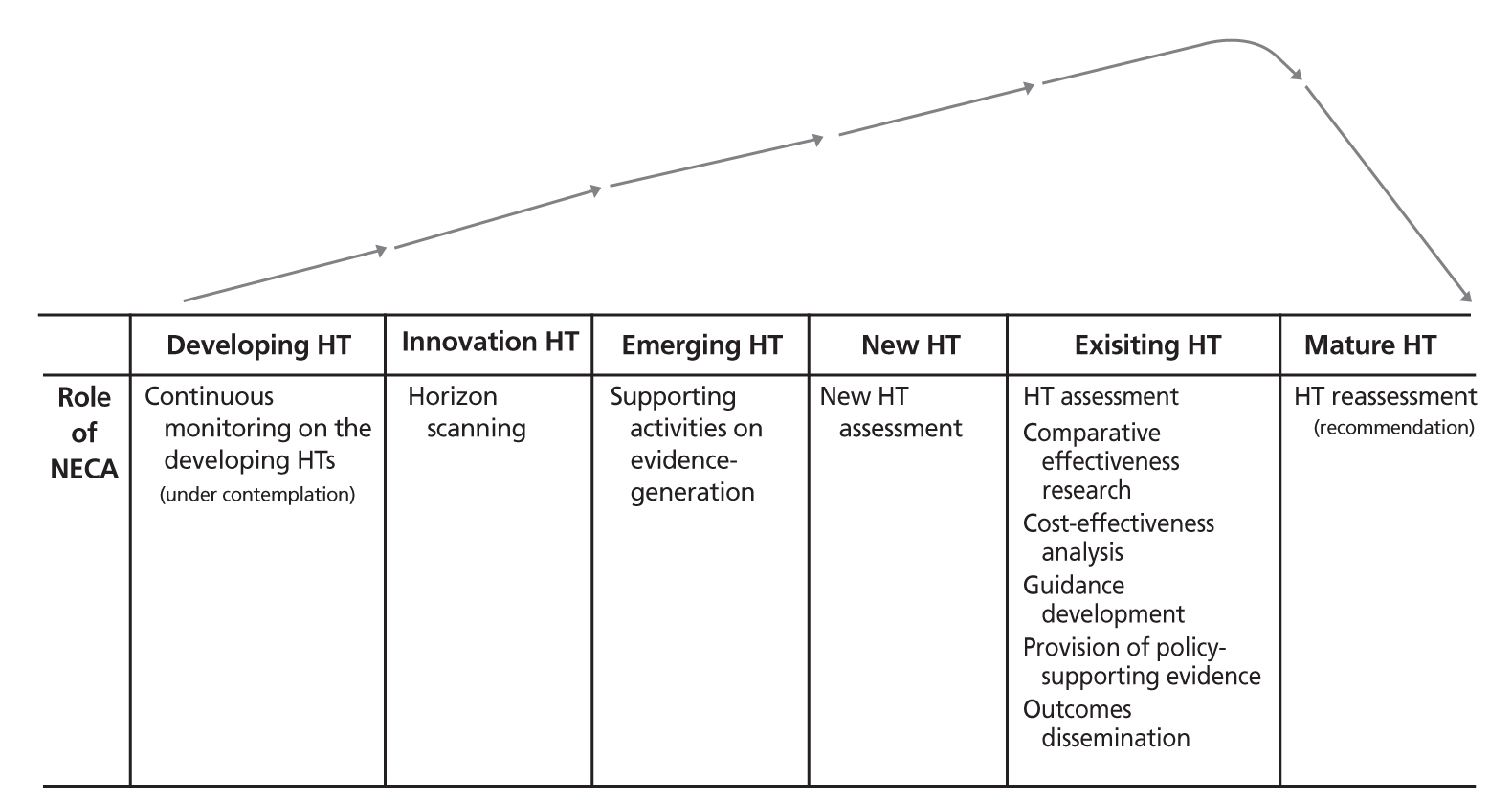
- Health technology assessment was first introduced to the Republic of Korea in 2006 by amending the Medical Services Act. The Committee of New Health Technology Assessment (CNHTA) is the ministerial committee that has the responsibility of reviewing the safety and effectiveness of new health technology. CNHTA review plays a gatekeeping role for new health technology in Korea, which can increase the burden on patients in Korea, either by out-of pocket payments or co-pays for National Health Insurance covered service. This kind of gatekeeping is a function of the healthcare system in many countries where no financial cap such as a fixed budget or diagnosis-related group payment is applied. However, it has been argued that gatekeeping works against industrial promotion policy. The one-stop service introduced in 2014 is a system similar to US parallel review between the US Food and Drug Administration and Centers for Medicare and Medicaid Services. This service provides a simultaneous process of regulatory review by the Ministry of Food and Drug Safety, identification of existing technology by the Health Insurance Review and Assessment Services, and new health technology assessment by the National Evidence-based Healthcare Collaborating Agency and the Ministry of Health and Welfare. This service is expected to reduce the total review process by 3 to12 months. A limited health technology appointment service was introduced in April 2014. This service designates orphan health technologies and health technologies for rare and incurable diseases and supports evidence development at designated hospitals. Several countries have similar systems: US Coverage with Evidence Development, Canadian Conditionally Funded Field Evaluation, UK Only in Research, and many others. The future direction of Health technology assessment should focus on the life cycle management of health technology. A consistent, continuous, and transformative mechanism to manage from the research and development of health technology to delisting obsolete technology to make room for new innovative technology is warranted.
Keyword
MeSH Terms
-
Biomedical Technology*
Budgets
Centers for Medicare and Medicaid Services (U.S.)
Child
Child, Orphaned
Delivery of Health Care
Diagnosis-Related Groups
Financial Management
Gatekeeping
Humans
Insurance, Health
Korea
Life Cycle Stages
National Health Programs
Republic of Korea
United States Food and Drug Administration
Figure
Cited by 2 articles
-
Managing health technologies
Tae-Hwan Lim
J Korean Med Assoc. 2014;57(11):904-905. doi: 10.5124/jkma.2014.57.11.904.Principles for evaluating the clinical implementation of novel digital healthcare devices
Seong Ho Park, Kyung-Hyun Do, Joon-Il Choi, Jung Suk Sim, Dal Mo Yang, Hong Eo, Hyunsik Woo, Jeong Min Lee, Seung Eun Jung, Joo Hyeong Oh
J Korean Med Assoc. 2018;61(12):765-775. doi: 10.5124/jkma.2018.61.12.765.
Reference
-
1. Lee SM. A study on the system and management plan for health technology assessment. Seoul: Health Insurance Review Agency;2005.2. Choi YS. Uninsured medical fee status and management plan. Seoul: National Health Insurance Corporation;2007.3. Kim JY. Health technology promotion government R&D plan. Seoul: Korea Health Industry Development Institute;2014.4. US Food and Drug Administration. FDA-CMS parallel review [Internet]. New Hampshire: US Food and Drug Administration;2014. cited 2014 Nov 3. Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/ucm255678.htm.5. Trueman P, Grainger DL, Downs KE. Coverage with Evidence Development: applications and issues. Int J Technol Assess Health Care. 2010; 26:79–85.
Article6. Goeree R, Chandra K, Tarride JE, O'Reilly D, Xie F, Bowen J, Blackhouse G, Hopkins R. Conditionally funded field evaluations: PATHs coverage with evidence development program for Ontario. Value Health. 2010; 13:Suppl 1. S8–S11.
Article7. Shin CM. The development of clinical management guide for temporary approval of new health technology. Seoul: National Evidenced-based Healthcare Collaborating Agency;2013.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Priority survey between indicators and analytic hierarchy process analysis for green chemistry technology assessment
- Current status and future direction of digital health in Korea
- The Korean Health Care Delivery System Early in the 21st Century
- The Business Analysis on the Chronic Disease Management in PublicHealth Centers for the Electronic Health Records System
- The current status of the Korean student health examination