J Korean Orthop Assoc.
2011 Aug;46(4):273-281. 10.4055/jkoa.2011.46.4.273.
The Effect of Cefazolin on Mechanical Properties and Antibacterial Reactions of Calcium Phosphate Cement
- Affiliations
-
- 1Department of Orthopedic Surgery, Asan Medical Center, College of Medicine, University of Ulsan, Korea. shlee2@amc.seoul.kr
- 2Department of Orthopedic Surgery, Kangnam Asan Hospital, Seoul, Korea.
- 3Department of Orthopedic Surgery, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea.
- KMID: 2185424
- DOI: http://doi.org/10.4055/jkoa.2011.46.4.273
Abstract
- PURPOSE
The purpose of this study is to evaluate the change of mechanical properties and the effect of antibacterial reactions in calcium phosphate cement (CPC) mixed with cefazolin.
MATERIALS AND METHODS
We made CPC and a sodium alginate solution and we mixed in variable dosages of cefazolin and then we made a standard sized cement mold. With that we performed compression stress tests, drug releasing tests and antibacterial tests.
RESULTS
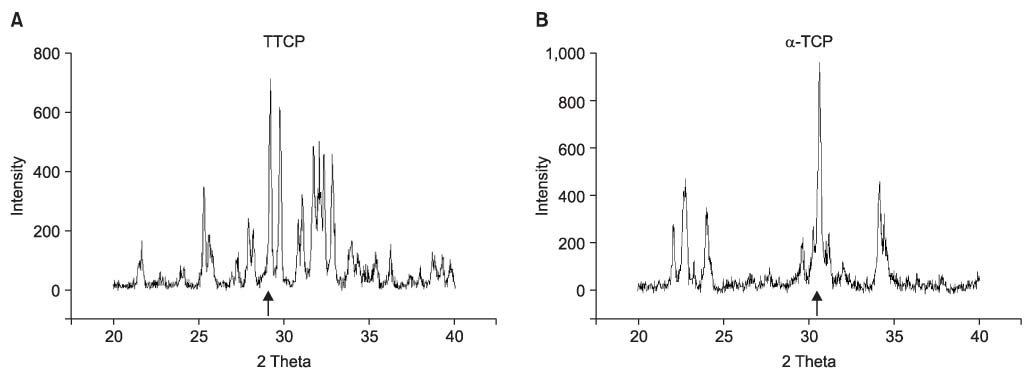
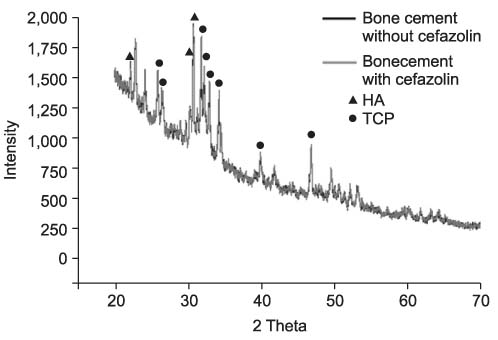
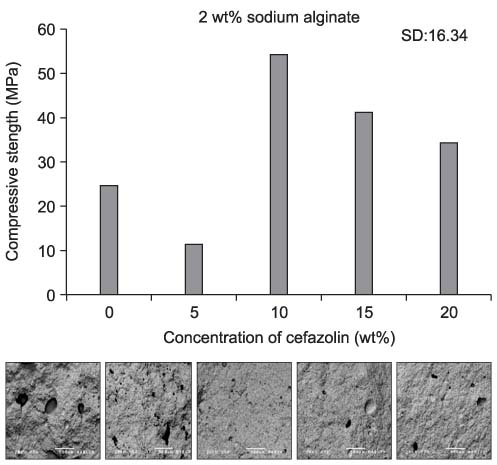
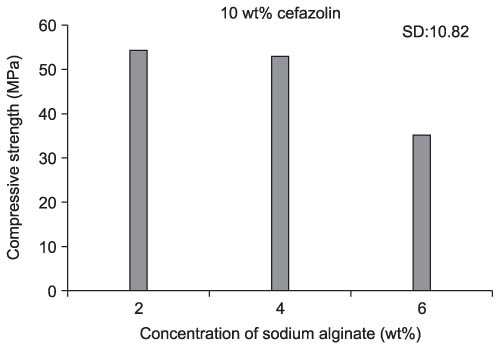
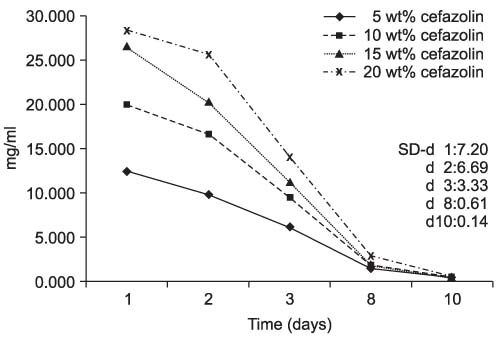
We found the typical appearance of hydroxyapatite (HA) in the cement mixed with cefazolin. The compressive strength of the cement mixed with cefazolin was higher than that of the cement not mixed with cefazolin and the higher strength cement had a smaller pore size and less porosity. The sodium alginate solution showed the maximum compressive strength at 2 & 4 wt%, but this was decreased at 6 wt%. Cefazolin was released in proportion to the concentration for the first 8 days on the drug releasing test and then a similar amount was released until the tenth day. An antibacterial effect was detected at all dosages of cefazolin on the antibacterial test.
CONCLUSION
The compressive strength of the cement mixed with cefazolin was higher than that of the cement not mixed with cefazolin. The drug was released from the cement in a proper fashion and the antibacterial effect was preserved.
MeSH Terms
Figure
Reference
-
1. Davies JP, Harris WH. Optimization and comparison of three vacuum mixing systems for porosity reduction of Simplex P cement. Clin Orthop Relat Res. 1990. 254:261–269.
Article2. Bruijn JD, Seelen JL, Feenstra RM, Hansen BE, Bernoski FP. Failure of the Mecring screw-ring acetabular component in total hip arthroplasty. A three to seven-year follow-up study. J Bone Joint Surg Am. 1995. 77:760–766.
Article3. Armstrong MS, Spencer RF, Cunningham JL, Gheduzzi S, Miles AW, Learmonth ID. Mechanical characteristics of antibiotic-laden bone cement. Acta Orthop Scand. 2002. 73:688–690.
Article4. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2003. 217:9–12.
Article5. Dunne NJ, Hill J, McAfee P, Kirkpatrick R, Patrick S, Tunney M. Incorporation of large amounts of gentamicin sulphate into acrylic bone cement: effect on handling and mechanical properties, antibiotic release, and biofilm formation. Proc Inst Mech Eng H. 2008. 222:355–365.
Article6. He Y, Trotignon JP, Loty B, Tcharkhtchi A, Verdu J. Effect of antibiotics on the properties of poly(methylmethacrylate)-based bone cement. J Biomed Mater Res. 2002. 63:800–806.
Article7. Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed Engl. 2002. 41:3130–3146.
Article8. Ambard AJ, Mueninghoff L. Calcium phosphate cement: review of mechanical and biological properties. J Prosthodont. 2006. 15:321–328.
Article9. Comuzzi L, Ooms E, Jansen JA. Injectable calcium phosphate cement as a filler for bone defects around oral implants: an experimental study in goats. Clin Oral Implants Res. 2002. 13:304–311.
Article10. Davies JP, O'Connor DO, Burke DW, Harris WH. Influence of antibiotic impregnation on the fatique life of Simplex P and Palacos R acrylic bone cements, with and without centrifugation. J Biomed Mater Res. 1989. 23:379–397.11. Davies JP, O'Connor DO, Greer JA, Harris WH. Comparison of the mechanical properties of Simplex P, Zimmer Regular, and LVC bone cements. J Biomed Mater Res. 1987. 21:719–730.
Article12. Ratier A, Gibson IR, Best SM, Freche M, Lacout JL, Rodriguez F. Setting characteristics and mechanical behaviour of a calcium phosphate bone cement containing tetracycline. Biomaterials. 2001. 22:897–901.
Article13. Ratier A, Freche M, Lacout JL, Rodriguez F. Behaviour of an injectable calcium phosphate cement with added tetracycline. Int J Pharm. 2004. 274:261–268.
Article14. Duncan CP, Masri BA. The role of antibiotic-loaded cement in the treatment an infection after a hip replacement. J Bone Joint Surg Am. 1994. 76:1742–1751.15. Webb JC, Spencer RF. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J Bone Joint Surg Br. 2007. 89:851–857.
Article16. Carter DR, Gates EI, Harris WH. Strain-controlled fatigue of acrylic bone cement. J Biomed Mater Res. 1982. 16:647–657.
Article17. Charnley J. Acrylic cement in orthopaedic surgery. 1970. Baltimore: Williams and Wilkins.18. Buchholz HW, Engelbrecht H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg. 1970. 41:511–515.19. Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am. 1990. 72:574–581.
Article20. Costantino PD, Friedman CD, Jones K, Chow LC, Sisson GA. Experimental hydroxyapatite cement cranioplasty. Plast Reconstr Surg. 1992. 90:174–185.
Article21. Ikenaga M, Hardouin P, Lemaître J, Andrianjatovo H, Flautre B. Biomechanical characterization of a biodegradable calcium phosphate hydraulic cement: a comparison with porous biphasic calcium phosphate ceramics. J Biomed Mater Res. 1998. 40:139–144.
Article22. Davies JP, Burke DW, O'Connor DO, Harris WH. Comparison of the fatigue characteristics of centrifuged and uncentrifuged Simplex P bone cement. J Orthop Res. 1987. 5:366–371.
Article23. Davies JP, O'Connor DO, Burke DW, Jasty M, Harris WH. The effect of centrifugation on the fatigue life of bone cement in the presence of surface irregularities. Clin Orthop Relat Res. 1988. 229:156–161.
Article24. Jasty M, Davies JP, O'Connor DO, Burke DW, Harrigan TP, Harris WH. Porosity of various preparations of acrylic bone cements. Clin Orthop Relat Res. 1990. 259:122–129.
Article25. Gates EI, Carter DR, Harris WH. Comparative fatigue behavior of different bone cements. Clin Orthop Relat Res. 1984. 189:294–299.
Article26. Gates EI, Carter DR, Harris WH. Tensile fatigue failure of acrylic bone cement. J Biomech Eng. 1983. 105:393–397.
Article27. Hendriks JG, van Horn JR, van der Mei HC, Busscher HJ. Backgrounds of antibiotic-loaded bone cement and prosthesis-related infection. Biomaterials. 2004. 25:545–556.
Article28. Ishikawa K, Asaoka K. Estimation of ideal mechanical strength and critical porosity of calcium phosphate cement. J Biomed Mater Res. 1995. 29:1537–1543.
Article29. Carlsson AS, Gentz CF. Mechanical loosening of the femoral head prosthesis in the Charnley total hip arthroplasty. Clin Orthop Relat Res. 1980. 147:262–270.
Article30. Elizabeth DH, Laurie BH, John RH, John CR. Increased killing of staphylococci and calcium phosphate hydraulic cement. J Biomed Mater Res. 1998. 140:139–144.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The experimental study of the effect of zinc phosphate cement on the solubility of enamel
- A Study on The Antibacterial Effect of antibiotic Impregnated Bone Cement
- Characterization and antimicrobial efficacy of Portland cement impregnated with silver nanoparticles
- Two Cases of Cement Burn
- A Case of Cement Burn