J Gynecol Oncol.
2013 Oct;24(4):359-366. 10.3802/jgo.2013.24.4.359.
Feasibility, acceptability and preferences for intraperitoneal chemotherapy with paclitaxel and cisplatin after optimal debulking surgery for ovarian and related cancers: an ANZGOG study
- Affiliations
-
- 1Department of Medical Oncology, Concord Repatriation General Hospital, Sydney, Australia. prunella.blinman@sswahs.nsw.gov.au
- 2NHMRC Clinical Trials Centre, University of Sydney, Sydney, Australia.
- 3Department of Obstetrics and Gynaecology, The Royal Hobart Hospital, Hobart, Australia.
- 4Department of Gynaecological Oncology, Mercy Hospital for Women, Melbourne, Australia.
- 5Flinders Centre for Innovation in Cancer, Flinders Medical Centre/Flinders University, Bedford Park, Australia.
- 6Department of Oncology, Christchurch Hospital, Christchurch, New Zealand.
- 7Department of Gynaecological Oncology, Westmead Hospital and University of Sydney, Sydney, Australia.
- 8Cancer Services, Mater Adult Hospital, Brisbane, Australia.
- 9Sydney Cancer Centre, Royal Prince Alfred and Concord Hospitals, Sydney, Australia.
- 10Department of Medical Oncology, Prince of Wales Hospital, Sydney, Australia.
- KMID: 2177878
- DOI: http://doi.org/10.3802/jgo.2013.24.4.359
Abstract
OBJECTIVE
Intraperitoneal (IP) chemotherapy in women with optimally debulked stage III ovarian cancer has been reported to prolong overall survival, but has not been widely adopted due to concerns about its toxicity, inconvenience and acceptability to patients. The purposes of this study were to determine the regimen's feasibility, adverse events, catheter-related complications, progression-free survival, health-related quality of life (HRQL), and patients' preferences for IP versus intravenous (IV) chemotherapy.
METHODS
We conducted a single arm, multi-center study of IP chemotherapy with IV paclitaxel 135 mg/m2 (D1) over 3 hours, IP cisplatin 75 mg/m2 (D2), and IP paclitaxel 60 mg/m2 (D8) for 6 cycles in women with optimally debulked stage III ovarian or related cancers.
RESULTS
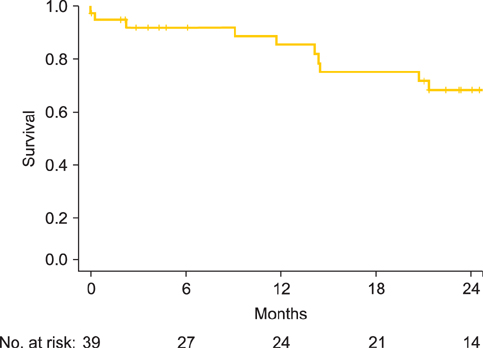
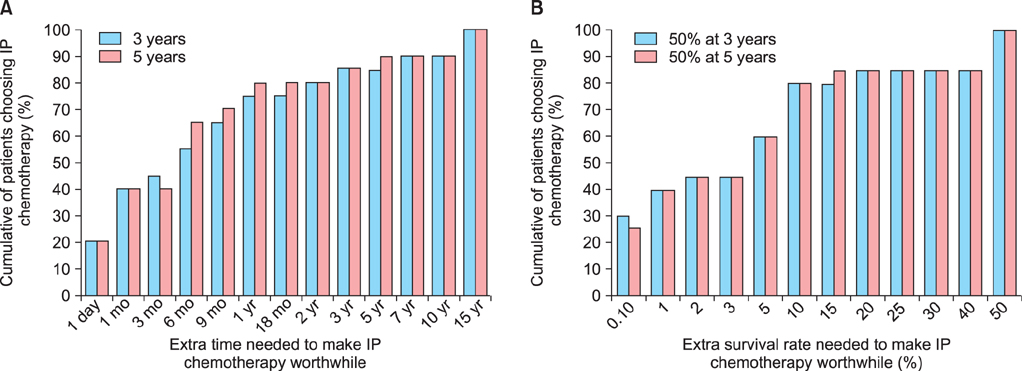
Thirty-eight eligible patients were recruited from 12 sites between July 2007 and December 2009. Seventy-one percent (n=27) completed at least 4 cycles and 63% (n=24) completed all 6 cycles. Grade 3 or 4 adverse events included nausea (n=2), vomiting (n=2), abdominal pain (n=2), and diarrhea (n=1), but not febrile neutropenia, neurotoxicity, or nephropathy. There were no treatment-related deaths. Catheter-related complications were the most frequent cause of early discontinuation of treatment (16 patients, 21%). Apart from neurotoxicity HRQL which worsened over time, HRQL was stable or improved with time. Most patients (> or =50%) judged moderate benefits (e.g., an extra 6 months survival time or a 5% improvement in survival rates) necessary to make IP chemotherapy worthwhile.
CONCLUSION
IP chemotherapy was feasible, tolerable, and most participants considered moderate survival benefits sufficient to warrant the adverse effects and inconvenience.
MeSH Terms
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer;2010.3. du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003; 95:1320–1329.4. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–3200.5. Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009; 27:1419–1425.6. Cannistra SA. Cancer of the ovary. N Engl J Med. 2004; 351:2519–2529.7. Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996; 335:1950–1955.8. Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001; 19:1001–1007.9. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006; 354:34–43.10. Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2011; (11):CD005340.11. Hess LM, Benham-Hutchins M, Herzog TJ, Hsu CH, Malone DC, Skrepnek GH, et al. A meta-analysis of the efficacy of intraperitoneal cisplatin for the front-line treatment of ovarian cancer. Int J Gynecol Cancer. 2007; 17:561–570.12. Cancer Therapy Evaluation Program. NCI clinical announcement on intraperitoneal therapy for ovarian cancer [Internet]. Cancer Therapy Evaluation Program;2006. cited 2012 Jul 28. Available from: http://ctep.cancer.gov/highlights/20060105_ovarian.htm.13. Australian Institute of Health and Welfare. Australian cancer incidence and mortality (ACIM) books [Internet]. Canberra: Australian Institute of Health and Welfare;2012. cited 2013 Aug 7. Available from: http://www.aihw.gov.au/acim-books/.14. Blinman P, King M, Norman R, Viney R, Stockler MR. Preferences for cancer treatments: an overview of methods and applications in oncology. Ann Oncol. 2012; 23:1104–1110.15. Slevin ML, Stubbs L, Plant HJ, Wilson P, Gregory WM, Armes PJ, et al. Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ. 1990; 300:1458–1460.16. Duric V, Stockler M. Patients' preferences for adjuvant chemotherapy in early breast cancer: a review of what makes it worthwhile. Lancet Oncol. 2001; 2:691–697.17. Blinman P, Duric V, Nowak AK, Beale P, Clarke S, Briscoe K, et al. Adjuvant chemotherapy for early colon cancer: what survival benefits make it worthwhile? Eur J Cancer. 2010; 46:1800–1807.18. Duric VM, Stockler MR, Heritier S, Boyle F, Beith J, Sullivan A, et al. Patients' preferences for adjuvant chemotherapy in early breast cancer: what makes AC and CMF worthwhile now? Ann Oncol. 2005; 16:1786–1794.19. Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, Webster K, Cella D, Hu S, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001; 19:1809–1817.20. Stockler MR, O'Connell R, Nowak AK, Goldstein D, Turner J, Wilcken NR, et al. Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression: a placebo-controlled double-blind randomised trial. Lancet Oncol. 2007; 8:603–612.21. Cancer Therapy Evaluation Program. Common terminology criteria for adverse events v3.0 (CTCAE) [Internet]. Cancer Therapy Evaluation Program;2006. cited 2013 Aug 7. Available from: http://http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.22. Rustin GJ. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003; 21:10 Suppl. 187s–193s.23. Fujiwara K. Three ongoing intraperitoneal chemotherapy trials in ovarian cancer. J Gynecol Oncol. 2012; 23:75–77.24. Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, Gianni L, Myles J, van der Burg ME, et al. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol. 1994; 12:2654–2666.25. Barlin J, Dao F, Zgheib NB, Ferguson S, Sabbatini P, Hensley M, et al. Progression-free and overall survival of a modified outpatient regimen of primary intravenous/intraperitoneal paclitaxel and intraperitoneal cisplatin is comparable to results from GOG 172. Gynecol Oncol. 2012; 125:Suppl 1. S36–S37.26. Barlin JN, Dao F, Zgheib NB, Ferguson SE, Sabbatini PJ, Hensley ML, et al. Progression-free and overall survival of a modified outpatient regimen of primary intravenous/intraperitoneal paclitaxel and intraperitoneal cisplatin in ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2012; 125:621–624.27. Bunting M, Chan W, Brand A, Blomfield P. Intraperitoneal chemotherapy for advanced epithelial ovarian malignancy: lessons learned. Aust N Z J Obstet Gynaecol. 2009; 49:667–671.28. Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D. Gynecologic Oncology Group. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007; 25:437–443.29. Stiggelbout AM, de Haes JC. Patient preference for cancer therapy: an overview of measurement approaches. J Clin Oncol. 2001; 19:220–230.30. Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010; 363:943–953.31. Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011; 365:2473–2483.32. Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011; 365:2484–2496.33. Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009; 374:1331–1338.34. Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013; 14:1020–1026.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ovarian Squamous Cell Carcinoma Associated with Endometriosis: Poor Response to Chemotherapy
- Ovarian Malignant Mixed Mullerian Tumor Managed with Neoadjuvant Chemotherapy and Cytoreductive Surgery
- Intraoperative intraperitoneal chemotherapy with cisplatin in epithelial ovarian cancer
- Long-term survival after intraperitoneal chemotherapy with paclitaxel-cisplatin for recurrent primary peritoneal cancer resistant to multiple lines of intravenous chemotherapy
- A phase I dose-finding trial of hyperthermic intraperitoneal docetaxel combined with cisplatin in patients with advanced-stage ovarian cancer