J Gynecol Oncol.
2012 Apr;23(2):91-97. 10.3802/jgo.2012.23.2.91.
Intraoperative intraperitoneal chemotherapy with cisplatin in epithelial ovarian cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, CHA Gangnam Medical Center, CHA University School of Medicine, Seoul, Korea. sjseong@cha.ac.kr
- KMID: 2177581
- DOI: http://doi.org/10.3802/jgo.2012.23.2.91
Abstract
OBJECTIVE
To assess retrospectively the feasibility of intraoperative intraperitoneal (IP) chemotherapy with cisplatin in epithelial ovarian cancer.
METHODS
IP chemotherapy during optimal staging surgery was performed in 10 patients who were diagnosed with primary epithelial ovarian cancers between April 2008 and February 2011. Cisplatin (70 mg/m2 in 1 L normal saline solution) was administered in the abdominal cavity for 24 hours postoperatively and then adjuvant chemotherapy was started 2-4 weeks after surgery. Perioperative toxicity of the combined treatment was evaluated until the initiation of postoperative adjuvant chemotherapy.
RESULTS
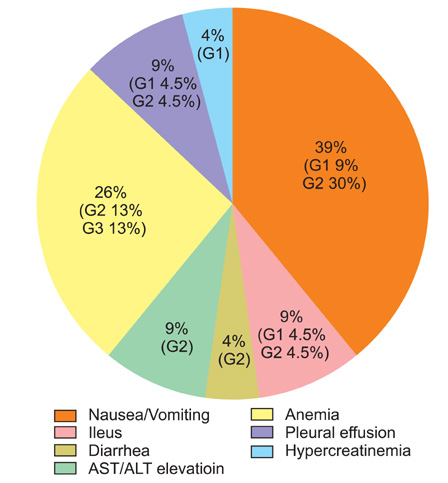
A total of 23 adverse events were observed in 9 of 10 patients (grade 1, 7; grade 2, 13; grade 3, 3; grade 4, 0). In descending order of frequency, adverse events affected the gastrointestinal system (n=14), hematologic system (n=6), pulmonary system (n=2), and genito-urinary system (n=1). The adverse events did not affect adjuvant systemic chemotherapy schedules. One patient experienced disease recurrence in the liver 16 months after surgery. The remaining 9 patients have been well controlled by chemotherapy and/or observation during the follow-up period of 4 to 39 months after surgery.
CONCLUSION
Intraoperative IP chemotherapy with cisplatin during surgical procedures is considered feasible for the treatment of primary epithelial ovarian cancer. Further studies, including long-term, prospective and comparative trials, are needed to validate the efficacy of this combined therapy.
MeSH Terms
Figure
Reference
-
1. Gadducci A, Carnino F, Chiara S, Brunetti I, Tanganelli L, Romanini A, et al. Intraperitoneal versus intravenous cisplatin in combination with intravenous cyclophosphamide and epidoxorubicin in optimally cytoreduced advanced epithelial ovarian cancer: a randomized trial of the Gruppo Oncologico Nord-Ovest. Gynecol Oncol. 2000. 76:157–162.2. Yen MS, Juang CM, Lai CR, Chao GC, Ng HT, Yuan CC. Intraperitoneal cisplatin-based chemotherapy vs. intravenous cisplatin-based chemotherapy for stage III optimally cytoreduced epithelial ovarian cancer. Int J Gynaecol Obstet. 2001. 72:55–60.3. Bijelic L, Jonson A, Sugarbaker PH. Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol. 2007. 18:1943–1950.4. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006. 354:34–43.5. Gonzalez-Moreno S, Gonzalez-Bayon LA, Ortega-Perez G. Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010. 2:68–75.6. Frenel JS, Leux C, Pouplin L, Ferron G, Berton-Rigaud D, Bourbouloux E, et al. Oxaliplatin-based hyperthermic intraperitoneal chemotherapy in primary or recurrent epithelial ovarian cancer: a pilot study of 31 patients. J Surg Oncol. 2011. 103:10–16.7. Yan TD, Zappa L, Edwards G, Alderman R, Marquardt CE, Sugarbaker PH. Perioperative outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy for non-appendiceal peritoneal carcinomatosis from a prospective database. J Surg Oncol. 2007. 96:102–112.8. Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001. 19:1001–1007.9. NCI clinical announcement: intraperitoneal chemotherapy for ovarian cancer [Internet]. National Cancer Institute. 2006. cited 2012 Mar 1. Bethesda, MD: National Cancer Institute;Available from: http://ctep.cancer.gov/highlights/docs/clin_annc_010506.pdf.10. Markman M, Walker JL. Intraperitoneal chemotherapy of ovarian cancer: a review, with a focus on practical aspects of treatment. J Clin Oncol. 2006. 24:988–994.11. Kim SW, Kim YT, Kim JW. New paradigm of intraperitoneal chemotherapy in ovarian carcinoma. Korean J Gynecol Oncol. 2006. 17:1–14.12. Walker JL, Armstrong DK, Huang HQ, Fowler J, Webster K, Burger RA, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2006. 100:27–32.13. Lim MC, Kang S, Choi J, Song YJ, Park S, Seo SS, et al. Hyperthermic intraperitoneal chemotherapy after extensive cytoreductive surgery in patients with primary advanced epithelial ovarian cancer: interim analysis of a phase II study. Ann Surg Oncol. 2009. 16:993–1000.14. Dovern E, de Hingh IH, Verwaal VJ, van Driel WJ, Nienhuijs SW. Hyperthermic intraperitoneal chemotherapy added to the treatment of ovarian cancer: a review of achieved results and complications. Eur J Gynaecol Oncol. 2010. 31:256–261.15. Chua TC, Robertson G, Liauw W, Farrell R, Yan TD, Morris DL. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol. 2009. 135:1637–1645.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for ovarian cancer
- Survival outcomes and toxicity of intraoperative intraperitoneal chemotherapy in advanced epithelial ovarian cancer
- Intraperitoneal cisplatin chemotherapy for advanced ovarian cancer
- Advanced ovarian cancer: what should be the standard of care?
- A Comparative study of Cyclophophamide and Cisplatin with or without Doxorubicin in Ovarian Carcinoma