J Gynecol Oncol.
2013 Oct;24(4):321-329. 10.3802/jgo.2013.24.4.321.
Utility of serum squamous cell carcinoma antigen levels at the time of recurrent cervical cancer diagnosis in determining the optimal treatment choice
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Osaka University Graduate School of Medicine, Osaka, Japan. smabuchi@gyne.med.osaka-u.ac.jp
- 2Department of Biomedical Statistics, Osaka University Graduate School of Medicine, Osaka, Japan.
- KMID: 2177873
- DOI: http://doi.org/10.3802/jgo.2013.24.4.321
Abstract
OBJECTIVE
To investigate the utility of serum squamous cell carcinoma antigen (SCC-Ag) levels upon the diagnosis of recurrent cervical cancer for decision making in patient management.
METHODS
Clinical records from 167 cervical cancer patients who developed recurrence between April 1996 and September 2010 were reviewed. A Cox proportional hazards regression model was used to investigate the prognostic significance of serum SCC-Ag levels at the time of recurrence. The effects of various salvage treatments on survival outcomes of recurrent cervical cancer were examined with respect to serum SCC-Ag levels.
RESULTS
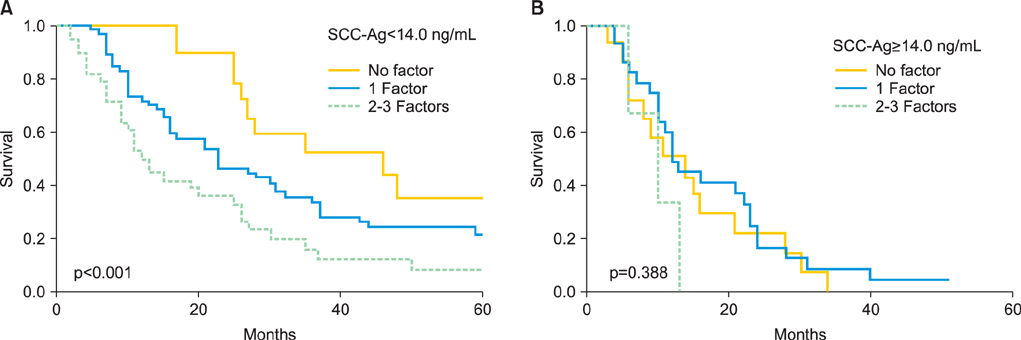
Serum SCC-Ag levels were elevated (>2.0 ng/mL) in 125 patients (75%) when recurrence was diagnosed. These patients exhibited significantly shorter postrecurrence survival than those with normal SCC-Ag levels (log-rank; p=0.033). Multivariate analyses revealed that an elevated serum SCC-Ag level was an independent prognostic factor for poor postrecurrence survival. In patients with SCC-Ag levels <14.0 ng/mL, radiotherapy or surgery resulted in improved survival compared with chemotherapy or supportive care. In contrast, in patients with SCC-Ag levels of > or =14.0 ng/mL, salvage treatment with radiotherapy had only a minimal impact on postrecurrence survival.
CONCLUSION
The serum SCC-Ag level measured when cervical cancer recurrence is diagnosed can be useful for deciding upon the appropriate salvage treatment.
MeSH Terms
Figure
Cited by 1 articles
-
Optimal cutoff level of serum squamous cell carcinoma antigen to detect recurrent cervical squamous cell carcinoma during post-treatment surveillance
Jinju Oh, Jin Young Bae
Obstet Gynecol Sci. 2018;61(3):337-343. doi: 10.5468/ogs.2018.61.3.337.
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. Ajiki W, Tsukuma H, Oshima A. Research Group for Population-based Cancer Registration in Japan. Cancer incidence and incidence rates in Japan in 1999: estimates based on data from 11 population-based cancer registries. Jpn J Clin Oncol. 2004; 34:352–356.3. Long HJ 3rd. Management of metastatic cervical cancer: review of the literature. J Clin Oncol. 2007; 25:2966–2974.4. Hockel M, Dornhofer N. Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. Lancet Oncol. 2006; 7:837–847.5. Fokdal L, Tanderup K, Nielsen SK, Christensen HK, Rohl L, Pedersen EM, et al. Image and laparoscopic guided interstitial brachytherapy for locally advanced primary or recurrent gynaecological cancer using the adaptive GEC ESTRO target concept. Radiother Oncol. 2011; 100:473–479.6. Powell ME. Modern radiotherapy and cervical cancer. Int J Gynecol Cancer. 2010; 20:11 Suppl 2. S49–S51.7. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009; 27:4649–4655.8. Omura GA, Blessing JA, Vaccarello L, Berman ML, Clarke-Pearson DL, Mutch DG, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1997; 15:165–171.9. Vermorken JB, Zanetta G, De Oliveira CF, van der Burg ME, Lacave AJ, Teodorovic I, et al. Randomized phase III trial of bleomycin, vindesine, mitomycin-C, and cisplatin (BEMP) versus cisplatin (P) in disseminated squamous-cell carcinoma of the uterine cervix: an EORTC Gynecological Cancer Cooperative Group study. Ann Oncol. 2001; 12:967–974.10. Mabuchi S, Isohashi F, Yoshioka Y, Temma K, Takeda T, Yamamoto T, et al. Prognostic factors for survival in patients with recurrent cervical cancer previously treated with radiotherapy. Int J Gynecol Cancer. 2010; 20:834–840.11. Mabuchi S, Matsumoto Y, Hamasaki T, Kawano M, Hisamatsu T, Mutch DG, et al. Elevated white blood cell count at the time of recurrence diagnosis is an indicator of short survival in patients with recurrent cervical cancer. Int J Gynecol Cancer. 2012; 22:1545–1551.12. Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977; 40:1621–1628.13. Maruo T, Shibata K, Kimura A, Hoshina M, Mochizuki M. Tumor-associated antigen, TA-4, in the monitoring of the effects of therapy for squamous cell carcinoma of the uterine cervix: serial determinations and tissue localization. Cancer. 1985; 56:302–308.14. Bolli JA, Doering DL, Bosscher JR, Day TG Jr, Rao CV, Owens K, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994; 55:169–173.15. Duk JM, de Bruijn HW, Groenier KH, Hollema H, ten Hoor KA, Krans M, et al. Cancer of the uterine cervix: sensitivity and specificity of serum squamous cell carcinoma antigen determinations. Gynecol Oncol. 1990; 39:186–194.16. Scambia G, Benedetti Panici P, Foti E, Amoroso M, Salerno G, Ferrandina G, et al. Squamous cell carcinoma antigen: prognostic significance and role in the monitoring of neoadjuvant chemotherapy response in cervical cancer. J Clin Oncol. 1994; 12:2309–2316.17. Takeda M, Sakuragi N, Okamoto K, Todo Y, Minobe S, Nomura E, et al. Preoperative serum SCC, CA125, and CA19-9 levels and lymph node status in squamous cell carcinoma of the uterine cervix. Acta Obstet Gynecol Scand. 2002; 81:451–457.18. Pras E, Willemse PH, Canrinus AA, de Bruijn HW, Sluiter WJ, ten Hoor KA, et al. Serum squamous cell carcinoma antigen and CYFRA 21-1 in cervical cancer treatment. Int J Radiat Oncol Biol Phys. 2002; 52:23–32.19. Ogino I, Nakayama H, Okamoto N, Kitamura T, Inoue T. The role of pretreatment squamous cell carcinoma antigen level in locally advanced squamous cell carcinoma of the uterine cervix treated by radiotherapy. Int J Gynecol Cancer. 2006; 16:1094–1100.20. Esajas MD, Duk JM, de Bruijn HW, Aalders JG, Willemse PH, Sluiter W, et al. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with early-stage cervical cancer. J Clin Oncol. 2001; 19:3960–3966.21. Yoon SM, Shin KH, Kim JY, Seo SS, Park SY, Kang S, et al. The clinical values of squamous cell carcinoma antigen and carcinoembryonic antigen in patients with cervical cancer treated with concurrent chemoradiotherapy. Int J Gynecol Cancer. 2007; 17:872–878.22. Forni F, Ferrandina G, Deodato F, Macchia G, Morganti AG, Smaniotto D, et al. Squamous cell carcinoma antigen in follow-up of cervical cancer treated with radiotherapy: evaluation of cost-effectiveness. Int J Radiat Oncol Biol Phys. 2007; 69:1145–1149.23. Mabuchi S, Okazawa M, Isohashi F, Matsuo K, Ohta Y, Suzuki O, et al. Radical hysterectomy with adjuvant radiotherapy versus definitive radiotherapy alone for FIGO stage IIB cervical cancer. Gynecol Oncol. 2011; 123:241–247.24. Hisamatsu T, Mabuchi S, Yoshino K, Fujita M, Enomoto T, Hamasaki T, et al. Prediction of progression-free survival and response to paclitaxel plus carboplatin in patients with recurrent or advanced cervical cancer. Int J Gynecol Cancer. 2012; 22:623–629.25. Kew FM, Roberts AP, Cruickshank DJ. The role of routine follow-up after gynecological malignancy. Int J Gynecol Cancer. 2005; 15:413–419.26. Chou HH, Wang CC, Lai CH, Hong JH, Ng KK, Chang TC, et al. Isolated paraaortic lymph node recurrence after definitive irradiation for cervical carcinoma. Int J Radiat Oncol Biol Phys. 2001; 51:442–448.27. Chan YM, Ng TY, Ngan HY, Wong LC. Monitoring of serum squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost-effective? Gynecol Oncol. 2002; 84:7–11.28. Brooks RA, Rader JS, Dehdashti F, Mutch DG, Powell MA, Thaker PH, et al. Surveillance FDG-PET detection of asymptomatic recurrences in patients with cervical cancer. Gynecol Oncol. 2009; 112:104–109.29. Chang TC, Law KS, Hong JH, Lai CH, Ng KK, Hsueh S, et al. Positron emission tomography for unexplained elevation of serum squamous cell carcinoma antigen levels during follow-up for patients with cervical malignancies: a phase II study. Cancer. 2004; 101:164–171.30. Sharma DN, Rath GK, Kumar R, Malhotra A, Kumar S, Pandjatcharam J, et al. Positron emission tomography scan for predicting clinical outcome of patients with recurrent cervical carcinoma following radiation therapy. J Cancer Res Ther. 2012; 8:23–27.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal cutoff level of serum squamous cell carcinoma antigen to detect recurrent cervical squamous cell carcinoma during post-treatment surveillance
- Clninical Utility of Serum Squamous Cell Carcinoma Antigen and Urine Polyamines in Cervical Carcinoma
- Relationship between Pre-treatment Serum SCC (squamous cell carcinoma) Antigen, Cyfra 21-1 Levels, and Survival in Squamous Cell Carcinoma of the Uterine Cervix
- The Clinical Significance of Squamous Cell Carcinoma Antigen as a Predictor of Nodal Metastasis in Early Stage Cervical Carcinoma
- The Evaluation of SCC (squamous cell carcinoma antigen) Level as a Tumor Marker in Patient with Squamous Cell Carcinoma of the Cervix