J Breast Cancer.
2014 Dec;17(4):376-385. 10.4048/jbc.2014.17.4.376.
Association between Pathological Complete Response and Outcome Following Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer Patients
- Affiliations
-
- 1Division of Medical Oncology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. minkonco@gmail.com
- 2Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
- KMID: 2176130
- DOI: http://doi.org/10.4048/jbc.2014.17.4.376
Abstract
- PURPOSE
We aimed to determine the rate of pathological complete response (pCR), clinicopathological factors associated with pCR, and clinical outcomes following neoadjuvant chemotherapy in locally advanced breast cancer.
METHODS
Medical records of patients who had undergone neoadjuvant chemotherapy for breast cancer between January 2007 and September 2011 were retrospectively reviewed, and the pCR rates were calculated according to three sets of criteria: the National Surgical Adjuvant Breast and Bowel Project (NSABP), the MD Anderson Cancer Center (MDACC), and the German Breast Group (GBG). Tumors were classified as luminal A like, luminal B like, human epidermal growth factor receptor 2 (HER2), or triple-negative. pCR and clinical outcome, including overall survival (OS) and disease-free survival (DFS) rates were analyzed at the median follow-up of 54.2 months.
RESULTS
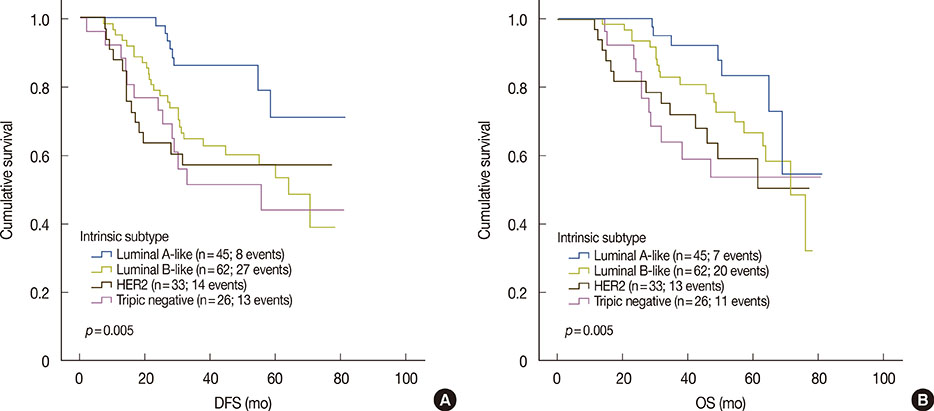
Of a total of 179 patients who had received neoadjuvant chemotherapy, 167 patients (93.3%) had locally advanced breast cancer and 12 patients (6.7%) had early-stage breast cancer. The majority of patients (152 patients, 89.4%) received anthracycline-based neoadjuvant chemotherapy. The objective clinical response rate was 61.5%, comprising clinical partial response in 5.5% and clinical complete response in 3.9% of patients. Twenty-one (11.7%), 20 (11.2%), and 17 patients (9.5%) achieved pCR according to NSABP, MDACC, and GBG definitions, respectively. pCR rates, as defined by NSABP, according to breast cancer subtype were 4.4%, 9.7%, 24.2%, and 19.2% in luminal A like, luminal B like, HER2, and triple-negative subtypes, respectively. Patients who achieved pCR had significantly better DFS (5-year DFS rates, 80% vs. 53%, p=0.030) and OS (5-year OS rates, 86% vs. 54%, p=0.042) than those who did not.
CONCLUSION
The pCR rate following neoadjuvant chemotherapy for breast cancer in Thai women attending our institution was 11.7%; pCR was more frequently observed in HER2 and triple-negative breast tumor subtypes. Patients who achieved pCR had significantly improved survival.
Keyword
MeSH Terms
-
Antineoplastic Combined Chemotherapy Protocols
Asian Continental Ancestry Group
Breast
Breast Neoplasms*
Disease-Free Survival
Drug Therapy*
Female
Follow-Up Studies
Humans
Medical Records
Neoadjuvant Therapy
Phenobarbital
Polymerase Chain Reaction
Receptor, Epidermal Growth Factor
Retrospective Studies
Treatment Outcome
Phenobarbital
Receptor, Epidermal Growth Factor
Figure
Reference
-
1. Ries LA, Young JL, Keel GE, Eisner MP, Lin YD, Horner MJ. SEER Survival Monograph: Cancer Survival among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program. Bethesda: National Cancer Institute;2007.2. Harris JR. Diseases of the Breast. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2010.3. Attasara P. Hospital-Based Cancer Registry 2010-2011. . Bangkok: National Cancer Institute, Department of Medical Services Ministry of Public Health Thailand;2011.4. Sachelarie I, Grossbard ML, Chadha M, Feldman S, Ghesani M, Blum RH. Primary systemic therapy of breast cancer. Oncologist. 2006; 11:574–589.
Article5. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998; 16:2672–2685.
Article6. Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002; 20:1456–1466.
Article7. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–2027.
Article8. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001; 19:4224–4237.
Article9. Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003; 21:4165–4174.
Article10. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–785.
Article11. Evans TR, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW, et al. Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: an Anglo-Celtic Cooperative Oncology Group study. J Clin Oncol. 2005; 23:2988–2995.
Article12. Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer. 2011; 47:2084–2090.
Article13. Ithimakin S, Ratanawichitrasin A, Veerasarn V, Akewanlop C, Soparattanapaisarn N, Rojananin S, et al. A phase II study of the combination of gemcitabine plus carboplatin as the neoadjuvant treatment in locally advanced breast cancer. J Med Assoc Thai. 2013; 96:Suppl 2. S67–S74.14. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747.
Article15. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.
Article16. Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, et al. Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer. 2002; 3:Suppl 2. S69–S74.
Article17. Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999; 17:460–469.
Article18. Miglietta L, Morabito F, Provinciali N, Canobbio L, Meszaros P, Naso C, et al. A prognostic model based on combining estrogen receptor expression and Ki-67 value after neoadjuvant chemotherapy predicts clinical outcome in locally advanced breast cancer: extension and analysis of a previously reported cohort of patients. Eur J Surg Oncol. 2013; 39:1046–1052.
Article19. Gupta D, Raina V, Rath GK, Shukla NK, Mohanti BK, Sharma DN. Clinical and pathological response rates of docetaxel-based neoadjuvant chemotherapy in locally advanced breast cancer and comparison with anthracycline-based chemotherapies: eight-year experience from single centre. Indian J Cancer. 2011; 48:410–414.
Article20. Krishnan Y, Alawadhi SA, P S S, Gopal M, Thuruthel S. Pathological responses and long-term outcome analysis after neoadjuvant chemotheraphy in breast cancer patients from Kuwait over a period of 15 years. Ann Saudi Med. 2013; 33:443–450.
Article21. Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004; 91:2012–2017.
Article22. von Minckwitz G, Raab G, Caputo A, Schütte M, Hilfrich J, Blohmer JU, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005; 23:2676–2685.
Article23. Untch M, Möbus V, Kuhn W, Muck BR, Thomssen C, Bauerfeind I, et al. Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol. 2009; 27:2938–2945.
Article24. Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005; 23:3676–3685.25. Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010; 28:2024–2031.
Article26. Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010; 375:377–384.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Clinical outcome and predictive factors for docetaxel and epirubicin neoadjuvant chemotherapy of locally advanced breast cancer
- Neoadjuvant Chemotherapy with Docetaxel and Adriamycin in Breast Cancer; Clincopathologic Factors Influencing to Response Rate
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Predictive and Prognostic Roles of Pathological Indicators for Patients with Breast Cancer on Neoadjuvant Chemotherapy