J Breast Cancer.
2014 Dec;17(4):339-343. 10.4048/jbc.2014.17.4.339.
Outcomes of Palliative Weekly Low-Dose Gemcitabine-Cisplatin Chemotherapy in Anthracycline- and Taxane- Pretreated Metastatic Breast Cancer Patients
- Affiliations
-
- 1Center for Breast Cancer, National Cancer Center, Goyang, Korea. parkinhae@gmail.com
- KMID: 2176124
- DOI: http://doi.org/10.4048/jbc.2014.17.4.339
Abstract
- PURPOSE
The combination of gemcitabine and cisplatin (GP) has been shown to be safe and efficacious for patients with metastatic breast cancer (MBC), pretreated with anthracyclines and taxanes. We assessed the efficacy and safety of weekly low-dose GP in patients with MBC.
METHODS
We collected clinicopathological data from MBC patients who had been treated with gemcitabine, 800 mg/m2 plus cisplatin, 30 mg/m2 intravenously, on days 1 and 8 every 3 weeks, between January 2001 and November 2011 in Korea.
RESULTS
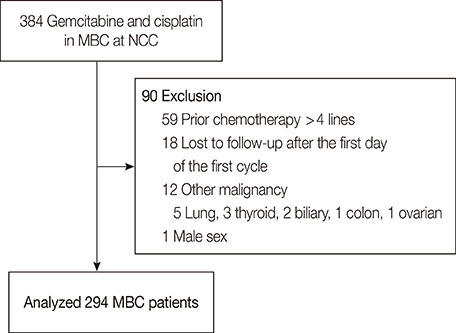
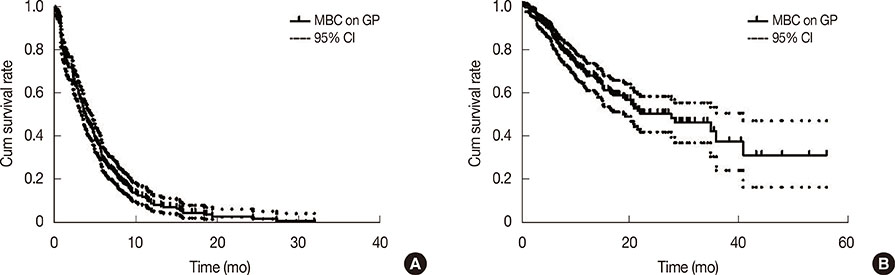
The analysis included 294 patients previously treated anthracycline-xand taxane-based chemotherapies prior to GP (median age, 48 years [range, 28-78 years]; median follow-up duration, 63.9 months). Seventeen patients (5.8%) discontinued GP because of toxicities. The median progression-free survival (PFS) was 3.9 months (95% confidence interval [CI], 3.394.4 months) and the median overall survival (OS) was 27.7 months (95% CI, 17.6-37.8 months) months. Statistically significant factors for PFS were performance status (Eastern Cooperative Oncology Group, > or =2 vs. <2; hazard ratio [HR], 1.37; 95% CI, 1.02-1.85; p=0.037), distant disease-free interval (DDFI; < or =2 years vs. >2 years; HR, 1.66; 95% CI, 1.28-1.95, p<0.001), time interval from the diagnosis of metastasis to GP therapy (< or =1 year vs. >1 year; HR, 1.48; 95% CI, 1.13-1.95, p<0.001), and presence of brain metastasis (HR, 1.47; 95% CI, 1.03-2.10; p=0.031). Similarly, DDFI (< or =2 years vs. >2 years; HR, 2.07; 95% CI, 1.36-3.14; p<0.001) and the presence of brain metastasis (HR, 2.14; 95% CI, 1.27-3.61; p=0.004) were important factors for OS after GP treatment.
CONCLUSION
Weekly low-dose GP chemotherapy appears safe and effective for heavily pretreated MBC patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.
Article2. Wirk B, Perez E. Role of gemcitabine in breast cancer management: an update. Semin Oncol. 2006; 33(1):Suppl 2. S6–S14.
Article3. De Laurentiis M, Cancello G, D'Agostino D, Giuliano M, Giordano A, Montagna E, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008; 26:44–53.
Article4. Ozkan M, Berk V, Kaplan MA, Benekli M, Coskun U, Bilici A, et al. Gemcitabine and cisplatin combination chemotherapy in triple negative metastatic breast cancer previously treated with a taxane/anthracycline chemotherapy; multicenter experience. Neoplasma. 2012; 59:38–42.
Article5. Rha SY, Moon YH, Jeung HC, Kim YT, Sohn JH, Yang WI, et al. Gemcitabine monotherapy as salvage chemotherapy in heavily pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005; 90:215–221.
Article6. Murphy CG, Seidman AD. Evolving approaches to metastatic breast cancer previously treated with anthracyclines and taxanes. Clin Breast Cancer. 2009; 9:Suppl 2. S58–S65.
Article7. Heinemann V. Gemcitabine plus cisplatin for the treatment of metastatic breast cancer. Clin Breast Cancer. 2002; 3:Suppl 1. 24–29.
Article8. Giovannetti E, Danesi R, Mey V, Nannizzi S, Pasqualetti G, Del Tacca M. In vitro studies on gemcitabine combinations with other antiblastics. Ann Oncol. 2006; 17:Suppl 5. v17–v19.
Article9. Sledge GW Jr, Loehrer PJ Sr, Roth BJ, Einhorn LH. Cisplatin as first-line therapy for metastatic breast cancer. J Clin Oncol. 1988; 6:1811–1814.
Article10. Koshy N, Quispe D, Shi R, Mansour R, Burton GV. Cisplatin-gemcitabine therapy in metastatic breast cancer: improved outcome in triple negative breast cancer patients compared to non-triple negative patients. Breast. 2010; 19:246–248.
Article11. Hastak K, Alli E, Ford JM. Synergistic chemosensitivity of triple-negative breast cancer cell lines to poly (ADP-Ribose) polymerase inhibition, gemcitabine, and cisplatin. Cancer Res. 2010; 70:7970–7980.
Article12. Seo JH, Oh SC, Choi CW, Kim BS, Shin SW, Kim YH, et al. Phase II study of a gemcitabine and cisplatin combination regimen in taxane resistant metastatic breast cancer. Cancer Chemother Pharmacol. 2007; 59:269–274.
Article13. Kim JH, Oh SY, Kwon HC, Lee S, Kim SH, Kim DC, et al. Phase II study of gemcitabine plus cisplatin in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Cancer Res Treat. 2008; 40:101–105.
Article14. Brito LG, de Andrade JM, Lins-Almeida T, Zola FE, Pinheiro MN, Marana HR, et al. Safety and efficacy of gemcitabine plus cisplatin combination in pretreated metastatic breast cancer patients. Med Oncol. 2012; 29:33–38.
Article15. Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005; 5:591–602.
Article16. Gonçalves A, Esterni B, Bertucci F, Sauvan R, Chabannon C, Cubizolles M, et al. Postoperative serum proteomic profiles may predict metastatic relapse in high-risk primary breast cancer patients receiving adjuvant chemotherapy. Oncogene. 2006; 25:981–989.
Article17. Wang T, Zhang S, Zeng M, Lu X, Shen G, Wu S, et al. Gemcitabine and cisplatin combination regimen in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Med Oncol. 2012; 29:56–61.
Article18. Sánchez-Escribano Morcuende R, Alés-Martínez JE, Aramburo González PM. Low dose gemcitabine plus cisplatin in a weekly-based regimen as salvage therapy for relapsed breast cancer after taxane-anthracycline-containing regimens. Clin Transl Oncol. 2007; 9:459–464.
Article19. Muss HB. Targeted therapy for metastatic breast cancer. N Engl J Med. 2006; 355:2783–2785.
Article20. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010; 363:1938–1948.
Article21. Erten C, Demir L, Somali I, Alacacioglu A, Kucukzeybek Y, Akyol M, et al. Cisplatin plus gemcitabine for treatment of breast cancer patients with brain metastases: a preferential option for triple negative patients? Asian Pac J Cancer Prev. 2013; 14:3711–3717.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase II Study of Gemcitabine plus Cisplatin in Patients with Anthracycline- and Taxane- Pretreated Metastatic Breast Cancer
- Treatment with Cisplatin and Etoposide Chemotherapy in Patient with Metastatic Breast Cancer
- Gemcitabine and Vinorelbine Combination Chemotherapy in Anthracycline- and Taxane-pretreated Advanced Breast Cancer
- Maintenance chemotherapy after 6 cycles of platinum-doublet regimen in anthracycline-and taxane-pretreated metastatic breast cancer
- Gemcitabine Single or Combination Chemotherapy in Post Anthracycline and Taxane Salvage Treatment of Metastatic Breast Cancer: Retrospective Analysis of 124 Patients